Structure-Hybridization
advertisement

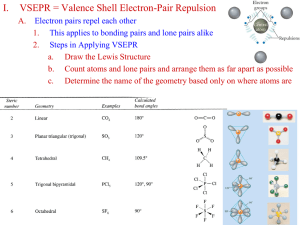
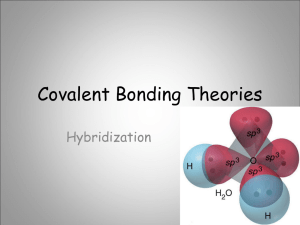
Valence Bond Theory and Hybridization Linus Pauling • Author: “Nature of the chemical bond” • Received Nobel prize in 1954 for his work • Introduced concept of orbital hybridization Valence bond theory – the basic idea Two half-filled orbitals overlap to form a covalent bond. The electrons in this new probability density are then shared by both atoms (equally attracted to both nuclei). Arrange themselves to have maximum overlap of their half filled orbitals, producing a bonding orbital of lowest energy! Two s orbitals overlapping Other overlaps – all form covalent bonds - all overlapping orbitals must be halffilled - the new probability distribution formed can only have a max of two electrons Types of covalent bonds • Orbitals can overlap in two main ways creating two different types of covalent bonds • Sigma bonds & Pi bonds The σ bond Electron density is between the nuclei of the overlapping atoms Single bonds σ bonds The π bond Electron densities are above and below the nuclei of the bonding atoms Only p orbitals can form pi bonds! Double Bond one π bond & one σ bond Triple bond two π bond & one σ bond The benzene ring Promotion & Hybridization • Certain atoms can change their electron configuration in order to bond and form a wide variety of compounds • This “change” in electron configuration takes place in two steps: Promotion of an electron to a higher energy orbital & hybridization or blending of orbitals creating a new type of orbital for bonding Promotion • Most of the time atoms exist in their “ground state” but, in certain cases the instant before bonding promotion takes place allowing more bonding spaces: Hybridization The merging of orbitals •Merging orbitals must all be half filled •No orbitals are “lost” due to merging – if you blend one s orbital and one p orbital you will end up with TWO hybrid orbitals! Hybridization Continued • Mix at least 2 nonequivalent atomic orbitals (eg. s and p). Hybrid orbitals have different shapes from original atomic orbitals • Covalent bonds are formed by: – Overlap of hybrid orbitals with atomic orbitals – Overlap of hybrid orbitals with other hybrid orbitals Example: Hybridization in carbon to form methane (CH4) Types & Names of hybrid orbitals • The type of hybrid orbital depends upon the orbitals which have been blended sp2 Hybridization in BF3 _ _ _ ↓ 2s2 2p1 Unhybridized Boron For BF3, 3 hybrid orbitals are needed, so 3 atomic orbitals are required as follows: (s + p + p) = sp2 _ sp2 _ sp2 _ sp2 Hybridized Boron 3 sp2 orbitals needed to form 3 sigma bonds sp Hybridization in BeCl2 __ _ ↓ 2p Unhybridized Be 2s2 • For BeCl2, 2 hybrid orbitals are needed, so 2 atomic orbitals are required as follows: (s + p ) = sp _ _ sp sp Hybridized Be Once hybridization has occurred – hybridized orbitals are ready to bond – just like regular orbitals Bonding in hybridized orbitals Because of their shape, hybrid orbitals can only undergo sigma bonding. Shapes & Hybrids – a little trick :0) Hybridization Involving d Orbitals promote 3s 3p 3d unhybridized P atom P = [Ne]3s23p3 3s 3p 3d vacant d orbitals hybridize Ba F Be F P five sp3d orbitals F 3d Be F Be F Ba Trigonal bipyramidal degenerate orbitals (all EQUAL) Sigma and Pi Bonds • Single Bond = 1 sigma bond • Double Bond = 1 sigma bond and 1 pi bond • Triple Bond = 1 sigma bond and 2 pi bonds