File
advertisement

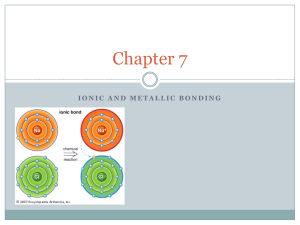
Chapter 7 Objectives-7.1 Determine the number of valence electrons in a representative element. Explain how the octet rule applies to atoms of metallic and nonmetallic elements. Describe how cations form. Explain how anions form. Review Describe what a valence electron is, and its location around the atom. Determine the valence electrons for Oxygen (AN=8), Cesium (AN=55), Neon (AN=10), and Bromine (AN=35) State the charge of the following elements when they become ions: Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne). How many valence electrons do the Noble Gases hold. (Excluding Helium) What are the names of the elements found in groups: IA, IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA? (1-8) Prior Knowledge We will be using much of the material we learned in chapter 6 for this unit. Valence electrons Ions Locations on the Periodic Table Valence Electrons Recalling from Chapter 6, Valence Electrons, are the electrons in the highest occupied energy level of an element’s atoms. There are two basic methods to discover the number of valence electrons an atom holds. First, we can produce an electron configuration Second we can look at the group that element is held in. The elements that are not valence, the blue electrons in the picture to the side, are called core electrons, electrons in all energy levels below the highest. KEYWORDS- CORE ELECTRONS Valence Electrons and Bonding When atoms form compounds, a bond is formed between the electrons. It is easier to form a bond with atoms that openings on their valence level. To help represent this we draw dots around the elements. This is called a Lewis Dot Structure, or an Electron Dot Structure. Your Turn Try and draw the Electron dot structures for the following elements. Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F). The Octet Rule We learned previously that noble gases are the most stable. Knowing that the noble gases are the most stable, because they contain a set number of valence electrons, all other elements strive to be like them. The Octet Rule By having the most stable elements all having equal numbers of valence electrons we discover a key concept. The octet rule, where when compounds are formed, atoms tend to achieve the electron configuration of noble gases. KEYWORD- OCTET RULE The Octet Rule Atoms of metals tend to lose their valence electrons, leaving a complete octet. Atoms in some non-metals tend to gain electrons, or share, with another nonmetal to achieve a complete octet. Formation of Cations and Anions As we have learned in the past Cations and Anions. Cations LOSE a negatively charged electron, and thus become positively charged. Anions GAIN a negatively charged electron, and thus become negatively charged. Formation of Transition Metal Ions The elements in the transition section of the periodic table, and some outside of it, have unique representations of ions. When a metal forms an ion we write is as follows: Fe3+, or Iron (III). When dealing with metals, roman numerals is equivalent to charge. Closing How do Cations Form? How do Anions Form? Non-metals tend to gain or lose electrons? Metals tend to gain or lose electrons? The octet rule states that a stable atom will have how many valence electrons?