Unit 1 Notes - Structure and Properties of Matter
advertisement

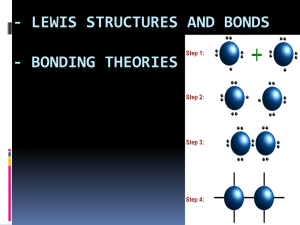
SCH4U – UNIT 1 STRUCTURE AND PROPERTIES CHAPTER 4 – CHEMICAL BONDING Activity • With a partner discuss everything you remember about chemical bonding • Eg. Types of bonds? why? What happens? 4.1 Types of Chemical Bonds • What are the two main types of chemical bonds? • Ionic: chemical bond between oppositely charged ions • Electrostatic attraction • Covalent: a chemical bond in which atoms share bonding electrons • Bonding Electron Pair: electron pair that is involved in bonding • Bond type depends on the attraction for electrons of the atoms involved • i.e. electronegativity Ionic Compounds How do these work? Metal + Non-Metal Metal+ + Non-MetalLow IE Low EA High IE High EA Isoelectronic with noble gases Opposites attract in no particular direction, considered nondirectional Ions cling together in clusters known as crystals • Get a lattice structure • Lattice energy: energy change when one mole of an ionic substance is formed from its gaseous ions • Depends on: • Charge on the ions • Size of the ions Ionic Compounds and Bonding • Properties – WHY? • Do not conduct electric current in the solid state • Conduct electric current in the liquid state • When soluble in water, form good electrolyte • Relatively high MP and B • Brittle, easily broken under stress Covalent Bonds Balance of attractive and repulsive forces What are the forces acting here? Octet Rule • Atoms share electrons so that they are surrounded by 8 electorns • # bonds = 8 - # valence electorn • Example: Carbon, Oxygen, Nitrogen • Two covalent bonds = double bond • Three covalent bonds = triple bond Lewis Structures • Atoms and ions are stable if they have a full valence shell • Electrons are most stable when they are pair • Atoms form chemical bonds to achieve full valence shells of electrons • Full valence shell may occur by an exchange or by sharing electrons • Sharing – covalent; exchange - ionic Polar Covalent Bonds • When electrons are shared unevenly in a covalent bond • Example: HF, H2O Coordinate Covalent Bonds • Both electrons are contributed by one atom • Example: • NH4+ • H3O+ • CO • N2O • NHO3 Resonance Structures • Single bonds are longer than double bonds, which are longer than triple bonds • Example: SO3 • Resonance Structure: Electron pair is shared over three bond evenly • Delocalized electrons Less than 8 • BeH2 • BCl3 More than 8 • Octet rule only applies to the first two periods • After that, can have expanded octets • Example: • PF5 • BrF5 • SiF63- Practice - Worksheet • H2 • F2 • OF2 • O2F2 Valence Bond Theory and Quantum Mechanics • Covalent bonds occur when orbitals overlap and two electrons occupy the same region of space • Example: H2 HF • What are the electron configurations for H and F? • How would the orbitals interact H2O • What are the electron configurations for H and O? • How would the orbitals interact Problem • We know from experiments in atomic structure that the bond angle in H2O is 104.5°… not 90° as predicted by valence bond theory • True for CH4 (109.5°) and NH3 (107.5°) – VBT always predicts bond angles of 90° • So, we need a better theory… Hybrization • Two problems still exist from Lewis Bonding Theory 1. Carbon atoms form 4 EQUAL C-H bonds in CH4 (or any other molecule) • Not predicted due to electron configuration of C • Recall: s orbitals have lower energy than p orbitals, therefore the bond length would be different 2. Existence of double and triple bonds Hybridization of Carbon Orbitals • An s electron gets promoted to the empty p-orbital • This stabilizes the p- and s- orbitals and gives them all the same energy; • Half-filled subshells • Called sp3 orbitals (HYBRID ORBITALS) • Each sp3 orbital lies at 109.5° Additional Hybrid Orbitals – sp - LINEAR Additional Hybrid Orbitals – sp2 - PLANAR Additional Hybrid Orbitals – sp3 Tetrahedral Double and Triple Bonds • Two types of orbital overlap exist • What we have seen so far is one type 1. Sigma bonds: σ-bonds • End-on-end overlap of orbitals 2. Pi bonds:π-bonds • Sideways overlap of orbitals Sigma Bonds • Occur in single bonds and account for the FIRST bond in a double or triple bond • Examples: Pi Bonds • Occur when p-orbitals not on the bonding axis (py or pz) overlap with each other Making Double Bonds • Example: C2H4 • Draw a Lewis Structure • What occurs with the C atoms hybridization? • For double bonds, there must be one σ-bond from overlapping hybrid orbitals and one π-bond from overlapping py or pz orbitals • Come from sp2 hybridized orbitals and result in trigonal planar structures Making Triple Bonds • Triple bonds have one σ-bond and two π-bonds; come from sp-hybridized orbitals, and result in linear structures • Central atom has two un-hybridized p-orbitals Practice • Explain the structure of the following molecules using electron configurations, orbital hybridization and VBT. • C2Cl4 • C2Cl2 • CO2 VSEPR Theory - Valence Shell Electron Pair Repulsion Theory Work through VSEPR Chart • Fun times with molecular structure… Practice Problems • Use Lewis Theory and VSEPR Theory to predict the structure of the following molecules: • Homework/Practice - Worksheet Polar Molecules • Polar molecules are molecules where the electron charge is not distributed evenly Electronegativity and Polar Covalent Bonds • Ionic Bond: ΔEN = >1.7 • Electron transfer • Polar Covalent Bond: ΔEN = 0.5-1.7 • Electrons shared unevenly • Pure Covalent Bond: ΔEN = 0.0-0.5 • Electrons shared evenly • Remember: Think of electrons as electron probabilities, electron cloud density is greater around one atom or another, therefore one gets a slight negative, the other slight positive charge • Think of the scale as a continuum Polar Molecules • Cannot exist if there are no polar bonds! • Bond dipole: electronegativity difference of two atoms represented by an arrow pointing from the positive to the negative end (lower to higher EN) • Non-polar molecule: either perfectly symmetrical so the bond dipoles cancel out, or when no polar bonds exist • Polar molecule: occur when bond dipoles do not cancelout • Example: • Determine the polarity of the following molecules • H2O, CCl4, NH3, PCl5 • Practice: • CH3Cl, BeCl2, SiO2, BrF4 • CHF2Cl • CH3NH2 Intermolecular Forces • Forces that exist between molecules • Three types: • Dipole-Dipole • Hydrogen Bonds • London Dispersion • In order to determine the Intermolecular Forces (IMF), you need to first determine the polarity of the molecule • Much weaker than covalent bonds Dipole-Dipole Forces • Occur in polar molecules • The slightly negative end on one molecule is attracted to the slightly positive end on another molecule • Strength depends on the size of the dipole London Dispersion Forces • Simultaneous attraction of the electrons in one molecule to the nuclei in the surrounding molecules • Increase as the number of electrons and protons in a molecule increase • Exist in ALL molecules • Weakest Force Hydrogen Bonds • Attraction between H on one molecule and O, N, or F on another molecule • Strongest of the intermolecular forces • Found in H2O, NH3, and HF, or whenever there is a –OH, -NH2 in a molecule Predicting Strength of IMF • Use pol Predicting Boiling Points • Boiling points increase as IMF strength increases • Arrange the following molecules in order of increasing boiling points 1. SiH4, SnH4, GeH4, CH4, 2. C3H8, C2H4, C4H10 3. CH4, CCl3H, CBr3H Practice • Determine the intermolecular forces that exist in each molecule • CCl4, C5H12, CH3CH2OH • Which molecules would have the strongest IMF • C2H5OH, C2H6, C2H5Cl • Explain you answer Structure and Properties of Solids • Different types of solids result depending on the type of bonding in the solid • These solids have different properties Ionic Crystals • Crystal Lattice • Properties result from the lattice structure • Brittle, high melting/boiling point, conduct electricity when dissolved in water or in liquid form, hard Molecular Crystals • Arrangement of neutral molecules held together by weak intermolecular forces • Properties vary depending on the strength of the IMF Covalent Network Solids • Array of covalently bonded atoms, structure is held together by covalent bonds • High MP/BP • Example: Silicate (SiO) Carbon Network Solids • Diamond, Graphite, Carbon Nanotubes, Buckminster Fullerenes • Explain the difference in properties between graphite and diamond. Metallic Crystals • Lots of electrons, but low ionization energy means they are loosely held • Lots of empty valence orbitals with similar energy, therefore electrons are free to move around • Strong, non-directional bonding Properties of Metals Property Shiny, Silvery Flexible Electrical Conductivity Hard Solids Crystalline Explanation End of UNIT 1 – STRUCTURE AND PROPERTIES!! • Your unit test is on: October 28 • Review package handed out