Hybrization of Orbitals
advertisement
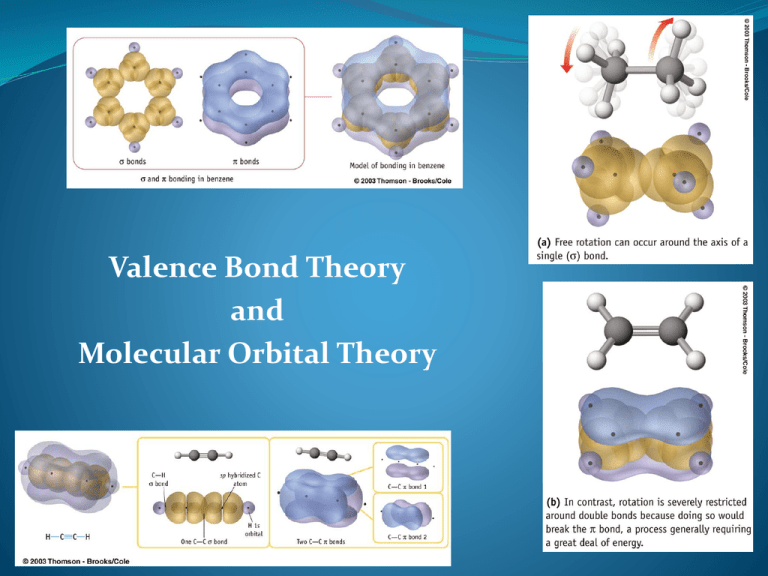
Valence Bond Theory and Molecular Orbital Theory Shapes of Atomic Orbitals for Electrons Four different kinds of orbitals for electrons denoted s, p, d, and f s and p orbitals most important in organic and biological chemistry s orbitals: spherical, nucleus at center p orbitals: dumbbell-shaped, nucleus at middle d orbitals: elongated dumbbell-shaped, nucleus at center 2 The Nature of Chemical Bonds: Covalent bond forms when two atoms approach each other closely so that a singly occupied orbital on one atom overlaps a singly occupied orbital on the other atom Two models to describe covalent bonding. Valence bond theory, Molecular orbital theory Central Themes of Valence Bond Theory Basic Principle of Valence Bond Theory: a covalent bond forms when the orbitals from two atoms overlap and a pair of electrons occupies the region between the nuclei. 1) Opposing spins of the electron pair. The region of space formed by the overlapping orbitals has a maximum capacity of two electrons that must have opposite spins. 2) Maximum overlap of bonding orbitals. The bond strength depends on the attraction of nuclei for the shared electrons, so the greater the orbital overlap, the stronger the bond. Central Themes of Valence Bond Theory 3) Hybridization of atomic orbitals. bonding in simple diatomic molecules: Example1: HF (direct overlap of the s and p orbitals of isolated ground state atoms). Example 2: CH4 (4 hydrogen atoms are bonded to a central carbon atom)- hybridization happens to obtain the correct bond angles. Pauling proposed that the valence atomic orbitals in the molecule are different from those in the isolated atoms. We call this Hybridization! Fig. 11.1 What Is A Hybrid Orbital? are a type of atomic orbital that results when two or more atomic orbitals of an isolated atom mix (the number of hybrid orbitals on a covalently bonded atom is equal to the number of atomic orbitals used to form the hybrid orbitals) are used to describe the orbitals in covalently bonded atoms (hybrid orbitals do not exist in isolated atoms), have shapes and orientations that are very different from those of atomic orbitals in isolated atoms in a set are equivalent, and form identical bonds (when the bonds are to a set of identical atoms), and are usually involved in sigma bonds in polyatomic molecules; pi bonds usually involve the overlap of unhybridized orbitals. In Hybridization there is ‘mixing’ or ‘blending’ of atomic orbitals to accommodate the spatial requirements in a molecule. Hybridization occurs to minimize electron pair repulsions when atoms are brought together to form molecules. Types of Hybrid Orbitals 1. sp3 Orbitals and the Structure of Methane Carbon has 4 valence electrons (2s2 2p2) In CH4, all C–H bonds are identical (tetrahedral) sp3 hybrid orbitals: s orbital and three p orbitals combine to form four equivalent, unsymmetrical, tetrahedral orbitals (sppp = sp3), Pauling (1931) 11 Carbon: 2s22px12py1 one electron in each of four sp3 The Structure of Methane sp3 orbitals on C overlap with 1s orbitals on 4 H atoms to form four identical C-H bonds Each C–H bond has a strength of 436 (438) kJ/mol and length of 109 pm Bond angle: each H–C–H is 109.5°, the tetrahedral angle. 13 The sp3 Hybrid Orbitals in NH3 and H2O Fig. 11.5 Hybridization of Nitrogen and Oxygen Elements other than C can have hybridized orbitals H–N–H bond angle in ammonia (NH3) 107.3° C-N-H bond angle is 110.3 ° N’s orbitals (sppp) hybridize to form four sp3 orbitals One sp3 orbital is occupied by two nonbonding electrons, and three sp3 orbitals have one electron each, forming bonds to H and CH3. 15 sp3 Orbitals and the Structure of Ethane Two C’s bond to each other by s overlap of an sp3 orbital from each Three sp3 orbitals on each C overlap with H 1s orbitals to form six C–H bonds C–H bond strength in ethane 423 kJ/mol C–C bond is 154 pm long and strength is 376 kJ/mol All bond angles of ethane are tetrahedral 16 2. sp2 Orbitals and the Structure of Ethylene sp2 hybrid orbitals: 2s orbital combines with two 2p orbitals, giving 3 orbitals (spp = sp2). This results in a double bond. sp2 orbitals are in a plane with 120° angles Remaining p orbital is perpendicular to the plane 17 Bonds From 2 sp Hybrid Orbitals Two sp2-hybridized orbitals overlap to form a s bond p orbitals overlap side-to-side to formation a pi () bond sp2–sp2 s bond and 2p–2p bond result in sharing four electrons and formation of C-C double bond Electrons in the s bond are centered between nuclei Electrons in the bond occupy regions are on either side of a line between nuclei 18 Structure of Ethylene H atoms form s bonds with four sp2 orbitals H–C–H and H–C–C bond angles of about 120° C–C double bond in ethylene shorter and stronger than single bond in ethane Ethylene C=C bond length 134 pm (C–C 154 pm) 19 Fig. 11.3 3. sp Orbitals and the Structure of Acetylene C-C a triple bond sharing six electrons Carbon 2s orbital hybridizes with a single p orbital giving two sp hybrids two p orbitals remain unchanged sp orbitals are linear, 180° apart on x-axis Two p orbitals are perpendicular on the y-axis and the z-axis 21 Orbitals of Acetylene Two sp hybrid orbitals from each C form sp–sp s bond pz orbitals from each C form a pz–pz bond by sideways overlap and py orbitals overlap similarly 22 Bonding in Acetylene Sharing of six electrons forms C C Two sp orbitals form s bonds with hydrogens 23 4. The sp3d Hybrid Orbitals in PCl5 Fig. 11.6 5. The sp3d2 Hybrid Orbitals in SF6 Sulfur Hexafluoride -- Fig. 11.7 SF6 Molecular Orbital Theory A molecular orbital (MO): where electrons are most likely to be found (specific energy and general shape) in a molecule Additive combination (bonding) MO is lower in energy Subtractive combination (antibonding) MO is higher energy 27 Molecular Orbitals in Ethylene The bonding MO is from combining p orbital lobes with the same algebraic sign The antibonding MO is from combining lobes with opposite signs Only bonding MO is occupied 28 Valence Bond Theory vs. MO Theory VB Theory begins with two steps: hybridization (where necessary to get atomic orbitals that “point at each other”) combination of hybrid orbitals to make bonds with electron density localized between the two bonding atoms Key differences between MO and VB theory: MO theory has electrons distributed over molecule VB theory localizes an electron pair between two atoms MO theory combines AOs on DIFFERENT atoms to make MOs (LCAO) VB theory combines AOs on the SAME atom to make hybridized atomic orbitals (hybridization) In MO theory, the symmetry (or antisymmetry) must be retained in each orbital. In VB theory, all orbitals must be looked at once to see retention of the molecule’s symmetry. 29 Sigma and Pi Bonds Difference between sigma and pi bond Sigma bond(σ) Formed by head to head overlap of AO’s The 2 AO’s that overlap are symmetrical about the x axis joining the 2 nuclei. Has free rotation Lower energy Only one bond can exist between two atoms( a single covalent bond) Pi bond(π) Formed by side-to-side overlap of AO’s No free rotation Has higher energy One or two bonds can exist between two atoms Have nodal plane on the molecular axis and no longer symmetrical about the molecular axis. Formation of Sigma Molecular Orbitals: 1. Overlapping of two 1s atomic orbital Example: H2 + bonding antibonding 2. Overlapping of two px atomic orbital bonding antibonding + 3. Overlapping of an s and px atomic orbitals bonding + antibonding 4. Overlapping of px and dz2 or dx2-y2 bonding antibonding Formation of Pi Molecular Orbitals: 1. Overlapping of two py atomic orbitals + bonding antibonding 2. Overlapping of two pz atomic orbitals + bonding antibonding 3. Overlapping of py or pz with dxz or dxy + bonding antibonding Fig. 11.10 Fig. 11.11 Comparison between sigma and pi electrons in ethylene and acetylene 1. Pi electron are made exposed to the environment than the sigma electrons. 2. Pi electrons are more reactive than sigma electrons. 3. The looseness of the pi electrons in C2H2 is less than in C2H4. This is the result of the greater s character in sp hybrids as compared with the s character in sp2 hydrid. 4. Pi electrons in C2H2 are attracted more strongly towards the nucleus than the pi electrons in C2H4. 5. The pi bonds in C2H2 are more susceptible to attack by other chemical entities than in C2H4. Valence Bond Theory: Overlap of Atomic Orbitals s bond - overlap of s orbitals 1s s bond - overlap of + F: H-F s orbital and p orbital 2p 1 s bond - head/head N2 N: overlap of p orbitals 2s 2p 2 bonds - sidewise Bonds with hybridization of atomic orbitals: overlap of p orbitals H-H sp3 hybridization CH4 H: 2s 2p C: 1s sp3 C atom: 4 s bonds - overlap of H-s orbitals and sp3 orbitals Of C A single sp3 orbital, each with single electron sp2 hybridization H2CO C: 2s H 2p H O H O p sp2 H C C atom 3 s bond; 1 bond A single sp2 orbital, each with single electron sp hybridization CO2 O C C: 2s 2p O O sp p C O C atom: 2 s bonds 2 bond A single sp orbital, each with single electron Dinitrogren: 2 Models N N without hybridization – with just p electrons N2 N: 2s 2p with sp hybridization – with all valence electrons N: N2 2s 2p p sp 1st sp orbital one with nonbonded pair 2p sp2 p -geometry around O is trigonal planar - requires 3 equivalent orbitals from the O - hence, sp2 hybridization N 1 s bonds 2 bond O H 2s 2nd sp orbital N O atom in H2CO (previous slide) O: 1 s bond - head/head overlap of p orbitals 2 bonds - sidewise overlap of p orbitals H O atom Can form 1 s bond; Can form 1 bond Has 2 nonbonded pairs





