sep22nd2011
advertisement

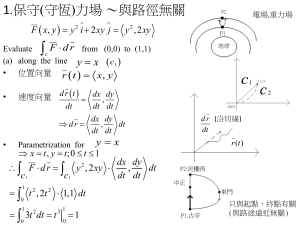
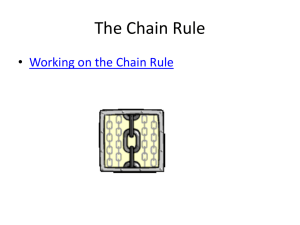
Chem 430 Particle on a ring 09/22/2011 Richard Feynman I think I can safely say that nobody understands quantum mechanics Quantum mechanics is based on assumptions and the wave-particle duality The nature of wave-particle duality is not known To explain and predict experimental results: (A) A quantum system has many possible states; (B) Each state has a well defined energy; (C) At anytime, the system can be in one or more states; (D) The probability in each state is determined by energy and other factors. What is energy ? In many cases, define probability The energy of each state will not change The system energy can change s c 0 ( t ) 0 c1 ( t ) 1 ... c n ( t ) n Energy value (frequency) is obtained from the oscillation of the coefficients Oscillating dipole generates electromagnetic radiation 0.10 CH3NCO -1 CH3NCO/2288 cm 0.8 0.08 0.6 PP signal Absorbance (normalized) 1.0 30cm-1 0.4 0.06 0.04 0.2 0.02 2200 2220 2240 2260 2280 2300 2320 2340 0.5 -1 Frenquency (cm ) 1.0 1.5 2.0 2.5 Delay time (ps) T c 1 3000 3 10 8 1.1 10 12 s 1.1 ps 3.0 3.5 4.0 Polar Coordinates (2D) x r cos y x r O y r sin P (x,y) y x 0 ~ 2 Cylindrical Coordinates (3D) z y x r P(x,y,z) y x x r cos y r sin z z Why use the new coordinates rather than the Cartesian Coordinates? Fewer variables, easier to calculate Variables can be separated because of symmetry Rotation a rotation is a rigid body movement which keeps a point fixed. a progressive radial orientation to a common point In Cartesian coordinates, two variables In polar coordinates, only one variable x r co s y r sin r is constant Particle on a ring Particle mass : m Potential:0 Radius: r=a=constant Angle: the only variable a General procedure Write down Hamiltonian Simplify Math with symmetry Use boundary conditions to define energy levels Particle on a ring Hamiltonian only contains the kinetic energy part 2 H 2 2 ( 2 m x 2m 2 2 2 y ) 2 In the polar coordinate system 2 2 x 2 2 y 2 2 r 2 1 r r 1 2 r 2 2 2 2 x 2 2 y 2 1 2 r 2 r r 2 x 2 r x r 2 2 r r x r 2 r r x ) x r r x ( x ( r ( 2 ( Need to eliminate 2 x r x r r x x x ) r r ) ( ) x x r x x x r 2 r r x ( r x x 2 The chain Rule y r sin 2 r x r co s Apply it twice 1 r r x 2 r x 2 x 2 0 2 2 x 2 ) r r x x 2 ) x x x r cos ; y r sin dx cos dr r sin d ; dy sin dr r cos d dr cos dx s in dy ; d sin cos dx r 2 x r 2 cos 2 r sin cos co s ; s in ; ; . x y x r y r r dy 2 r 2 2 sin cos r r 2 sin cos r 2 sin 2 r r sin r 2 2 2 2 y 2 sin 2 2 r 2 2 sin cos r r 2 sin cos r 2 cos 2 r r cos r 2 2 2 2 2 2 2 x 2 2 y 2 2 r 2 1 r r 1 2 r 2 2 http://en.wikibooks.org/wiki/Partial_Differential_Equations /The_Laplacian_and_Laplace's_Equation ra 2 H r 2 2 2m ( r 2 2 m r 2 2 H H d 0 2 1 r r I mr Moment of inertia 2 2 d 2 I d 2 2 r 2 2 2 ) 2mr 2 2 2 I d 2 2 E 1 d 2 d 2 2 IE 2 d 2 2 d 2 Ae ml ( im l 2 IE 2 Be im l 1 ) 2 E ml 2 2 2I Cyclic boundary condition P ( ) Q ( 2 ) On the ring, Points P = Q ( ) ( 2 ) Ae im l Be im l Ae im l e im l 2 Be im l e im l 2 Ae im l Be im l Ae im l e im l 2 Be im l e im l 2 e im l 2 cos m l 2 i sin m l 2 1 m l 0, 1, 2.... E ml ml 2 2 ; Ae im l 2I Why only choose one portion for the wave function? d Normalized * d A e Ae 2 im l 2 A 1 2 0 1 A * im l 2 1 2 1 2 e im l Constant for all angles Why? Why is it different from in the 1D box? Implications: (1) probability is same at any point (2) Position can’t be determined at all Consequence of arbitrary position No zero point energy m l 0, E 0 p 0; E 0, m l l Double degeneracy Particle can rotate clockwise or counterclockwise The Circular Square Well y a V (r a ) 0 V (r a ) x 2 H 2 2 2 m r 2 ( 2m ( r 2 2 1 r r 1 r r 1 2 r 2 1 2 ) 2 r 2 2 ) E C ircular sym m etry can be separated into angular and radia l parts ( r , ) R ( r ) ( ) The angular part is known from the above 2 d R 2 ( 2m dr 2 2 1 d R 2 r dr d R 2 ( 2m 1 d R dr 2 1 dR r d 2 2 ) r dr ) E R 2 R 1 d 2 2m r 2 d 2 E R Divided by R 2 2 ( d R 2mR 1 dr 2 2 (r R 2 d R dr 1 2 1 dR r ) 2 e im l ; d 2 2 m r 2mE dr Radial 2 ) r dr dR 1 d 2 2 d E 1 d 2 r 2 d 2 angular 2 2 d 2 m 2 l m 2 l 2 r 2 d R dr 2 r dr 2 d R dz 2 dR 1 dR 2mE 2 (1 z dz r Rm R 2 m z 2 l 2 2 l )R 0 w ith k 2 2mE 2 ; z kr Bessel’s Equation Chem 430 Particle in circular square well and 09/27/2011 If particle is confined in a ring, At 0K, what is the most probable location to find it? How about at high temperature? How to explain these in terms of QM? 1. Find out possible states 2. Find out the energy of each state 3. Find out the wave function of each state to obtain its spatial distribution of probability Nothing to do with rotation J m l ( kr ) ( 1 ( kr ) ml 2 ( 1) n n0 1 kr ) 2n 2 n !( n m l ) ! Boundary condition r a; J m l ( ka ) 0 The condition gives allowed k and therefore energy k 2 2mE 2 J m l ( kr ) ( 1 ( kr ) ml 2 ( 1) n0 n 1 kr ) (A) 2n 2 n !( n m l ) ! J 0 2 .4 0 5 0 ( J 0 ( kr ) ( 1) 1 2.405 r 2 n normalized (r , ) ( J 0 ( kr ) ( 1) n0 n 1 kr ) 1.181 a 2n 2 2 k 2 5.783 2m 2ma 2 1 2n ( ( 1) 1 2.405 r 2 n ) 2n a n !n ! n0 -1 2 n !n ! 0 1 r/a (B) J 0 5 .5 2 0 0 E k 2 2 30.471 2m ( J 0 ( kr ) ( 1) 1 5.520 r 2 n ) 2n 2ma 2 1 a n !n ! n0 (A) 2 ka 5 .5 2 0 1 0 E 0 ) ka 2 .4 0 5 a n !n ! n0 ml 0 2 J 0 ( ka ) 0 (C) 0 (B) (r , ) 2.749 a 2 ( ( 1) 1 5.520 r 2 n n0 ) 2n a n !n ! -1 0.0 0.5 1.0 r/a -1 0 2 4 6 8 10 (C) J 0 8 .6 5 4 0 ( J 0 ( kr ) ( 1) n 1 8.654 r 2 0 0 2 74.887 ) 2ma 2 1 2n 0 2 ( r , ) rdrd 1 2 a n !n ! n0 a E k 2m kr 2 2 ka 8 .6 5 4 (r , ) 4.320 a 2 ( n0 ( 1) n 1 8.654 r 2 a n !n ! Many more states are possible if kr is bigger ) 2n -1 0.0 0.5 r/a 1.0 J m l ( kr ) ( 1 ( kr ) ml 2 ( 1) 1 kr ) J 1 3.832 0 2n 2 ka 3 .8 3 2 2 n !( n m l ) ! n n0 E k 2 2 14.682 2m 2ma 2 1.0 ml 1 J 1 ( kr ) 1 2 ( kr ( 1) n n0 1 J 1 ( kr ) kr ) 2n 1 2 ( kr ( 1) 1 3.832 r ) 2n 0.5 2 a n !( n 1) ! n n0 2 n !( n 1) ! 0.0 (r , ) 1.963 a 1.0 2 e i 1 2 ( kr ( 1) 1 3.832 r ) 2n -0.5 2 a n !( n 1) ! n n0 -1.0 0.0 0.5 1.0 r/a 0.5 J 1 7.016 0 0.0 ka 7 .0 1 6 k 2m -0.5 -1.0 2 E 2 2 49.218 2ma 2 1.0 0 2 4 6 8 10 1 J 1 ( kr ) kr 2 ( kr ( 1) n n0 0.5 1 7.016 r 2n ) 2 a n !( n 1) ! 0.0 -0.5 (r , ) 3.534 a 2 e i 1 2 ( kr ( 1) n0 n 1 7.016 r ) 2n 2 a n !( n 1) ! -1.0 0.0 0.5 r/a 1.0 J m l ( kr ) ( 1 ( kr ) ml 2 ( 1) n n0 1 kr ) J 2 5 .1 3 6 0 2n 2 n !( n m l ) ! J 2 ( kr ) ( ( kr ) 2 2 ( 1) n0 n 1 J 2 ( kr ) ( kr ) E k 2 2 26.375 2m ml 2 1 2 ka 5 .1 3 6 2n 1 ( kr ) ( 1) 2 2 1 5.136 r (r , ) a 2 e 2 i n0 ( 1 ( kr ) 2 2 ( 1) 2n 2 a n !( n 2) ! n 2 n !( n 2) ! 2.759 ) n n0 1 5.136 r ) 2ma 2 1.0 0.5 2n 2 a n !( n 2) ! 0.0 -0.5 1.0 -1.0 0.0 0.5 0.5 J 2 8 .4 1 7 0 0.0 ka 8 .4 1 7 2 E k 2m -0.5 -1.0 1.0 r/a 2 2 70.850 2ma 2 1.0 0 2 4 6 8 10 J 2 ( kr ) ( kr (r , ) 4.322 a 2 e 2 i ( 1 kr ) 2 2 1 2 ( ( 1) ( kr ) ( 1) n0 n ) 2n 2 a n !( n 2) ! n n0 2 1 8.417 r 1 8.417 r 0.5 0.0 -0.5 2n ) 2 a n !( n 2) ! -1.0 0.0 0.5 r/a 1.0 Energy level J 0 ( kr ) 1.0 J 1 ( kr ) 0.5 J 2 ( kr ) 0.0 -0.5 -1.0 0 2 4 6 kr 8 10 0 1 2 3 4 5 ml 0 1 2 0 3 1 energy 5.78 14.68 26.38 30.47 40.71 49.22 Angular Momentum i j l r p x k z i y px py pz y z py pz lz x ( l z i y i ) y( i z px pz k x y px py l z xp y yp x z com ponant ( k ) j x i x ) 1 2 (x i e y y x ) i im l ml 2 e im l m l Spherical Polar Coordinates z P(x,y,z) r y x y x z x r sin co s y r sin sin z r co s 2 1 r r 2 r 2 r 1 r sin 2 sin 1 2 r sin 2 2 2