Chapter 1.1: Types of Matter
advertisement
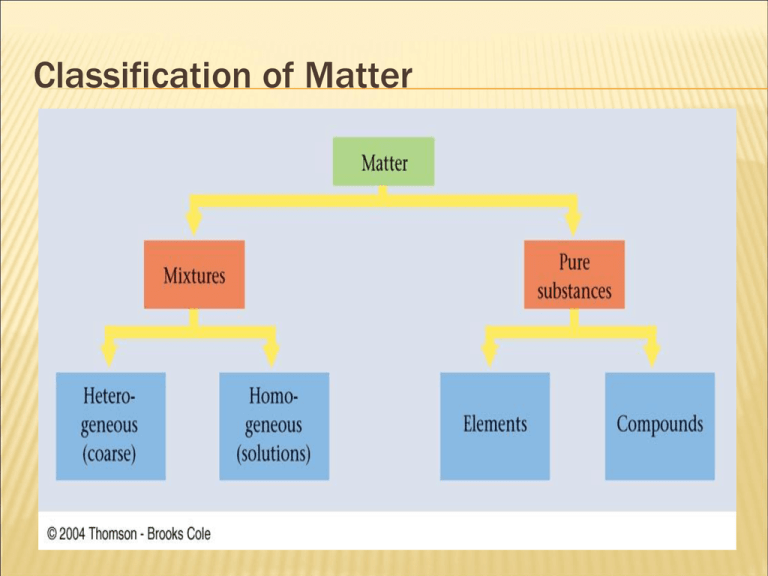
Classification of Matter CHART EXAMINING EACH SUBSTANCE OF AIR. 2 Measurement • Quantitative Science – – • Metric prefixes are often used Must memorize mega to micro SI Units – International units accepted in science for certain msmnts. • • • • • • Length – SI unit – Instrument Volume Mass Temperature - Accuracy - how close a measurements is to an accepted or true value. Precision - the ability to reproduce a measurement 5 • Rules – All numbers 1-9 are significant. • – Leading zeros are NEVER significant • – – Example: 0.00000000000000001 Captive (middle) zeros are always significant Trailing zeros are only significant when there is a decimal point. • – Example: 3,456 Example: 3,500 or 0.000025000 Middle zeros always count. • Example: 405 or 0.030600 Rules Greater than 5 round value up Less than 5 round value down If equal to 5, follow naked 5 rule: Even Stevens, the number remains the same Odd up, number rounds up Example: 2.35000, round to 2 sig figs. • These are large or small numbers that use a base 10 as a multiplying factor. – The number in front of the base 10 must be a number 1-9. • • Putting numbers into scientific notation – If you make the number smaller than the base 10 exponent gets larger by the number of places you move the decimal. Vice versa if you make the number larger (don’t forget sig figs): • • • Example: 660,00 = Example: .00066 = Taking numbers out of scientific notation – – • Example: 1.2 x 102 not 12 x 103 If exponent is negative make the number smaller (decimal to the left) If exponent is positive make the number larger (decimal to the right) Calculations – Multiplying/Dividing • – Plug into calculator directly – base 10’s do not need to be the same Adding/Subtracting • Units must be like terms and base 10’s need to be the same • Multiplying/Dividing: Least amount of sig figs – Units do not need to be the same • – When dividing variables with exponents subtract the exponents • – Example: 8 m3/ 2 m5 When multiplying variables with exponents add them • • Example: mi/hr or N*m Example: 3 km2 x 7 km4 Adding/Subtracting: Least amount of decimal places – Units must be like terms Example Problem: Sig Fig Calculations Carry out the following mathematical operations, and give each result with the correct # of sig figs: A. 1.05 x 10-3/ 6.135 B. 21 – 13.8 1.71 x 10-4 7 C. As part of a lab assignment to determine the value of the gas constant (R), a student measured the pressure, volume, and temperature for a sample of gas where R = PV/T. The following data was obtained: P = 2.560, T = 275.15, V = 8.8. Calculate the R constant. 8.4 x 10-2 Metric Prefixes Conversion Factor (key) Conversion Table Sample problem: How many micro-ounces are in 7.76 mg? 7.76 mg x 1 x 10-3g x 1 mg x 263 microoz 1kg x 2.12 lbs x 16 oz x 1microoz = 1 x 103 g x 1 kg x 1 lb x 1 x 10-6oz Example: Dimensional Analysis The latest model corvette has an engine with a displacement of 6.20 L. What is the displacement in units of cubic inches? 6.20 L x 1 ft3 x (12in)3 = 378 in3 28.32 L (1ft)3 THE THREE MAJOR TEMPERATURE SCALES. TK = TC + 273.15 TC = TK – 273.15 13 Converting Between Celsius & Fahrenheit More complex because both degree sizes and zero points are different. So two adjustments need to be made: One for the degree size Since 212*F = 100*C and 32*F = 0*C: 212 – 32 = 180 Fahrenheit degrees = 100 – 0 = 100 Celsius degrees 180*F or 9*F 100*C or 5*C or the reciprocal depending on which way you go. And one for the zero point Since 32*F = 0*C then we subtract 32 to find Celsius temp and add 32 to find Fahrenheit temp. TC = (TF – 32*F) 5*C/9*F TF = TC x 9*F/5*C + 32 Example Problem: Temperature Conersion Normal body temperature is 98.6 *F. Convert this temperature into the Celsius & Kelvin scales: 66.6*F x 5*C = 37.0*C 9*F TK = TC + 273.15 = 310.2 K Properties of Substances Observed without changing the chemical identity of a substance. Melting Point Freezing point Density = mass/volume Solubility = grams solute/100g water Color Observed only by changing chemical identity. Flammability Reactivity Ability to rust or tarnish Example: Density A chemist, trying to identify the main component of a compact disc cleaning fluid, finds that 25.00 cm3 of the substance has a mass of 19.625g at 20*C. The following densities (in g/mL) are given: chloroform: 1.492 ethanol: 0.789 toluene: 0.867 diethyl ether: 0.714 isopropyl alcohol: 0.785 Which of these is most likely to be the main component of the disc cleaner? D = m = 19.625 = 0.7850 g/cm3 V 25.00 Lab Instruments Lab Techniques Filtration Distillation Chromatography Spectrophotometry STM Filtration Vacuum Filtration SALT REMAINS AFTER ALL WATER IS BOILED OFF. PAPER CHROMATOGRAPHY Chromatography is a method used to separate mixtures. There is a mobile phase and a stationary phase. There are many types of chromatography TLC GC Gel Electrophoresis Spectrophotometer GAS CHROMATOGRAPHY STM & STM Images of Ni & Cs MC #1 A measured mass of an unreactive metal was dropped into a small graduated cylinder half filled with water. The following measurements were made. Mass of metal = 19.611 grams Volume of water before addition of metal = 12.4 milliliters Volume of water after addition of metal = 14.9 milliliters The density of the metal should be reported as (A) 7.8444 grams per mL (B) 7.844 grams per mL (C) 7.84 grams per mL (D) 7.8 grams per mL (E) 8 grams per mL MC #2 Mass of an empty container = 3.0 grams Mass of the container plus the solid sample = 25.0 grams Volume of the solid sample = 11.0 cubic centimeters The data above was gathered in order to determine the density of an unknown solid. The density of the sample should be reported as (A) 0.5 g/cm3 (B) 0.50 g/cm3 (C) 2.0 g/cm3 (D) 2.00 g/cm3 (E) 2.27 g/cm3 MC #3 Which of the following techniques is most appropriate for the recovery of solid KNO3 from an aqueous solution of KNO3? (A) Paper chromatography (B) Filtration (C) Titration (D) Electrolysis (E) Evaporation to dryness FRQ #1 The area of the 48 contiguous states is 3.02 x106 mi2. Assume that these states are completely flat (no mountains and no valleys). What volume of water, in liters, would cover these states with a rainfall of two inches? FRQ #2 Wire is often sold in pound spools according to the wire gauge number. That number refers to the diameter of the wire. How many meters are in a ten-pound spool of 12-gauge aluminum wire? A 12-gauge wire has a diameter of 0.0808 in. Aluminum has a density of 2.70 g/cm3. (V=π r2l) FRQ #3 Air is 21% oxygen by volume. Oxygen has a density of 1.31 g/L. What is the volume, in liters, of a room that holds enough air to contain 55 kg of oxygen? FRQ #4 54. The solubility of potassium chloride is 37.0 g/100 g water at 30°C. Its solubility at 70°C is 48.3 g/100 g water. (a) Calculate the mass of potassium chloride that dissolves in 48.6 g of water at 30°C. (b) Calculate the mass of water required to dissolve 52.0 g of potassium chloride at 70°C. (c) If 30.0 g of KCl were added to 75.0 g of water at 30°C, would it all disappear? If the temperature were increased to 70°C, would it then all dissolve? FRQ #5 A pycnometer is a device used to measure density. It weighs 20.455 g empty and 31.486 g when filled with water (d = 1.00 g/cm3). Pieces of an alloy are put into the empty, dry pycnometer. The mass of the alloy and pycnometer is 28.695 g. Water is added to the alloy to exactly fill the pycnometer. The mass of the pycnometer, water, and alloy is 38.689 g. What is the density of the alloy? FRQ #6 Describe a laboratory procedure needed to carry out each of the following. (a) Separate a mixture of powdered solid CaCl2 and CaCO3. (b) Determine the concentration of solute in an aqueous sodium chloride solution and give the concentration units that your method provides. (c) Separate a mixture of two volatile liquids. FRQ #7 Answer the following questions that relate to laboratory observations and procedures.. (a) An unknown gas is one of three possible gases: nitrogen, hydrogen, or oxygen. For each of the three possibilities, describe the result when the gas is tested using a glowing splint (a wooden stick with one end that has been ignited and extinguished, but still contains hot, glowing, partially burned wood). (b) The following three mixtures have been prepared: CaO plus water, SiO2 plus water, and CO2 plus water. For each mixture, predict whether it is basic, acidic, or neutral. Justify your answers. (c) Each of three beakers contains a 0.1 M solution of one of the following solutes: potassium chloride, silver nitrate, or sodium sulfide. Three beakers are labeled randomly as solution 1, solution 2, and solution 3. Shown below is a partially completed table of observations made of the results of combining small amounts of different pairs of the solutions. Sol 1 (i) Write the chemical formula of the black precipitate. (ii) Describe the expected results of mixing solution 1 with solution 3. (iii) Identify each of the solutions 1, 2 and 3. Sol 2 Sol 3 Sol 1 ----------- Black ppt ----------- Sol 2 ----------- ----------- No rxn Sol 3 ----------- ----------- -----------