Interfacial transport
advertisement

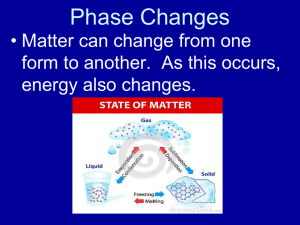
Interfacial transport • So far, we have considered size and motion of particles • In above, did not consider formation of particles or transport of matter between vapor and particulate phase • Interfacial transport – formation of aerosols by nucleation – growth by condensation – loss by evaporation Definitions • partial pressure - PA pressure that a vapor in a mixture of gases would exert if it were to occupy, (all by itself) the entire volume occupied by the mixture. • volume fraction of gas A = PA/Ptotal • saturation vapor pressure - PS if you had a sealed container containing liquid or solid A, the partial pressure of vapor phase A in equilibrium with the flat surface of liquid or solid at the T of the system • saturation ratio S = PA / PA, equilibrium also known as relative humidity for air/water systems Two types of nucleation • when the concentration of vapor is greater than the saturation vapor pressure, formation of the liquid or solid phase is thermodynamically favorable • homogeneous nucleation - condensation of a vapor takes place only on clusters of like molecules • heterogeneous nucleation - condensation occurs on a dissimilar cluster Energy balance on a newly forming particle G v L n d p 2 fv , f L = free - energy potential per molecule in vapor and liquid phase, in the drop, n total number of molecules contained g = surface tension In forming droplet, surface free energy went from zero to d2, a + contribution to free energy, but phase change of molecules to favored liquid phase is a (-) contribution to free energy. Imagine the partial pressure of the vapor near the droplet is changed by a small amount. droplet of size d in a supersaturated vapor. – After some substitutions and manipulations: 3 d N 2 p A G d p kT ln S p M 6 S saturation ratio N A Avogadro' s number M = molecular weight Shape of G vs dp Critical drop size, d* If another molecule is added by condensation, G will go down The Kelvin effect • curvature modifies attractive forces between surface molecules - the smaller the droplet, the easier it is for molecules to leave the surface • to maintain mass equilibrium, the equilibrium vapor pressure over a curved surface is greater than that for over a flat surface • Rearranging to solve for S, for droplets of diameter d*, the equilibrium vapor pressure over the droplet surface, pd, is given by: 4 M p d p S exp p RTd * Implications • A pure liquid drop will always evaporate when S < 1 • Even if supersaturation exists, droplets smaller than the critical size under those conditions will evaporate • Since smaller droplets (< d*) may evaporate under supersaturated conditions, large droplets may grow at the expense of small ones S - 0.9 Capillary condensation Kelvin equation in reverse!! Simulations for neck region S = 0.95 between nanoparticles using lattice gas stat thermo modeling. S=1 Seonmin Kim, graduate student in my group Homogeneous nucleation • even in unsaturated vapor, attractive forces between molecules lead to cluster formation, and a distribution of cluster sizes exists • with more vapor, this distribution shifts towards larger sizes • free energy of droplet is given by: NA 3 G d ( k T ln S ) d M 6 2 where = surface tension, d = droplet, M = molecular weight of liquid in drop, NA = Avogadro’s number, = droplet density More material - probability of larger clusters increases Homogeneous nucleation con’t • thermodynamics says that the system will go towards direction of decreasing free energy of system 4 M • recall * d RT ln S • for any given T, S, growth is favorable for clusters with d > d* (the critical nucleus diameter) • the greaterthe S, the smaller the critical nucleus diameter • rate of nucleation given by (“classical theory”): 2 N A M p G S exp RT kT 1/2 J d 2 p = saturation pressure over a plane of the liquid constant, usually taken as 1 kinetic -vs-activated nucleation • For some systems, S can be extremely high, and d* < diameter of a molecule • example: formation of refractory powders where chemical reaction is fast, and saturation vapor pressures are low • If this is the case, nucleation is said to be kinetic, limited only by rates of collisions between molecules, not by formation of clusters of critical size • nucleation discussed earlier - activated • kinetic nucleation can lead to some model simplifications Example problem: kinetic or activated? • consider silica at 1720 K, forming by rapid chemical reaction of a precursor in a flame • data: flame concentration of silica = 1 x 10-5 moles/liter flame gas at STP, 0.3 J /m2 surface tension, 60 g/mole, 2.2 g/cm3 density, equilibrium vapor pressure 4 x 10-9 bar Heterogeneous nucleation • how raindrops are formed- condensation of water vapor onto so called ‘condensation nuclei’ • heterogenous nucleation requires much lower saturation ratios than homogenous nucleation • free molecular growth - governed by rate of random molecular collisions between particle and vapor molecules • molecules may or may not stick, c is the fraction that stick, uncertainty as to the value (sometimes a value of 0.04 used) Growth laws for condensation • for growth in free molecular regime d d 2 M ( p p ) p dt p c o d 1/2 p N A (2 mkT ) o is partial pressure of vapor in gas surrounding droplet, pd is partial pressure of vapor at surface of droplet • for growth in the continuum regime, growth depends on rate of diffusion of droplet molecules to droplet surface Growth laws for condensation • rate of particle growth given by: (obtained for an isolated droplet) d d p dt where 2 D v M p o pd R p d p T Td = Fuch' s correction factor = 2 + d p d p 5 .33 / d p 3 .42 2 • correction factor is needed because diffusion equation breaks down within one mean free path of the surface, and growth becomes controlled by kinetic processes Sources of condensable species • Chemical reaction - if species formed has lower vapor pressure than precursor, and reaction rate is relatively fast compared to nucleation process • Physical - cooling via expansion or mixing with cold stream Aerosol formation and growth • to summarize: processes important for describing aerosol formation and growth – – – – nucleation condensation/evaporation coagulation coalescence An aerosol generator for production of metal nanoparticles 1.E+19 1.E+18 -1 1.E+16 -1 nuc le a t ion ra t e , log s c a le ( # k gg a s s ) 1.E+17 1.E+15 1.E+14 1.E+13 1.E+12 1.E+11 Indium 900C 1.E+10 1.E+09 Indium 1000C 1.E+08 1.E+07 Indium 1100C 1.E+06 1.E+05 1.E+04 1.E+03 1.E+02 1.E+01 1.E+00 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 distance (cm) Predicted nucleation rates as a function of distance accounting for nucleation, condensation, coagulation (not published) This assumes 1-D temp and velocity profiles in the tube 50