File - Loreto Science
advertisement
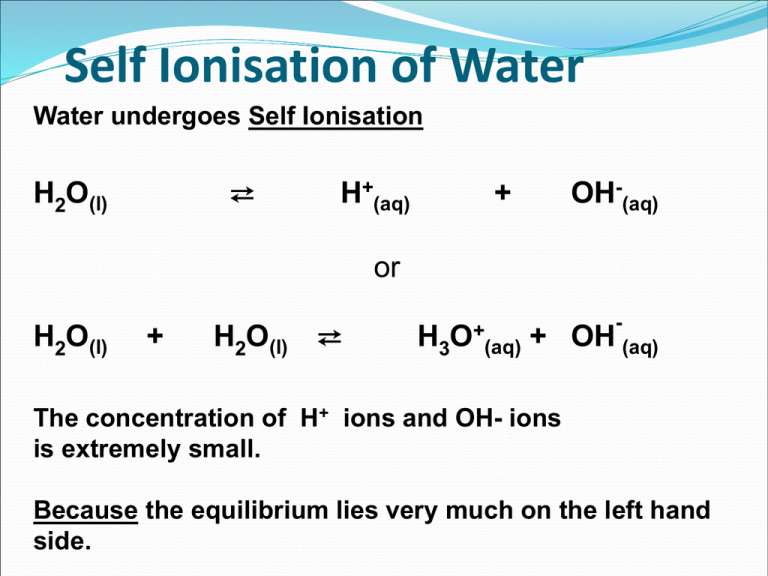
Self Ionisation of Water Water undergoes Self Ionisation ⇄ H2O(l) H+(aq) OH-(aq) + or H2O(l) + H2O(l) ⇄ H3O+(aq) - + OH (aq) The concentration of H+ ions and OH- ions is extremely small. Because the equilibrium lies very much on the left hand side. Glossary Ionisation Strong/weak acids Ionic Product Strong/Weak bases pH pH Curve Logarithm End-Point Kw Dissociation Constant Indicator pH scale Ionic Product of Water H2O(l) ⇄ H+(aq) + OH-(aq) Kc = In the above expression, the value of [H2O] may be taken as having a constant value because the degree of ionisation is so small. Kc = Kc [H2O] = [H+] [OH-] Both Kc and [H2O] are constant values so Kw = Kc [H2O] = [H+] [OH-] Kw = [H+] [OH-] is the ionic product of water Kw is temperature dependent T (°C) Kw (mol2/litre2) 0 0.114 x 10-14 10 0.293 x 10-14 20 0.681 x 10-14 25 1.008 x 10-14 30 1.471 x 10-14 40 2.916 x 10-14 50 5.476 x 10-14 Kw of pure water decreases as the temperature increases Acid–Base Concentrations in Solutions Acid–Base Concentrations in Solutions concentration (moles/L) 10-1 OH- H+ 10-7 H+ OH- OH- H+ 10-14 [H+] > [OH-] [H+] = [OH-] acidic solution neutral solution [H+] < [OH-] basic solution pH Scale Soren Sorensen (1868 - 1939) The pH scale was invented by the Danish chemist Soren Sorensen to measure the acidity of beer in a brewery. The pH scale measured the concentration of hydrogen ions in solution. The more hydrogen ions, the stronger the acid. The pH Scale 1 2 Strong Acid 3 4 Weak Acid 5 6 7 Neutral 8 9 10 11 Weak Alkali 12 13 14 Strong Alkali pH Scale The quantity of hydrogen ions in solution can affect the color of certain dyes found in nature. These dyes can be used as indicators to test for acids and alkalis. An indicator such as litmus (obtained from lichen) is red in acid. If base is slowly added, the litmus will turn blue when the acid has been neutralized, at about 6-7 on the pH scale. Other indicators will change color at different pH’s. A combination of indicators is used to make a universal indicator. Measuring pH Universal Indicator Paper Universal Indicator Solution pH meter Measuring pH pH can be measured in several ways Usually it is measured with a coloured acid-base indicator or a pH meter Coloured indicators are a crude measure of pH, but are useful in certain applications pH meters are more accurate, but they must be calibrated prior to use with a solution of known pH Limitations of pH Scale The pH scale ranges from 0 to 14 Values outside this range are possible but do not tend to be accurate because even strong acids and bases do not dissociate completely in highly concentrated solutions. pH is confined to dilute aqueous solutions pH At 250C Kw = 1 x 10-14 mol2/litre2 [H+ ] x [OH- ] = 1 x 10-14 mol2/litre2 This equilibrium constant is very important because it applies to all aqueous solutions - acids, bases, salts, and non-electrolytes - not just to pure water. For H2O(l) ⇄ → pH H+(aq) + OH-(aq) [H+ ] = [H+ ] x [OH- ] = 1 x 10-14 [OH- ] = [1 x 10-7 ] x [1 x 10-7 ] [H+ ] of water is at 250C is 1 x 10-7 mol/litre Replacing [H+ ] with pH to indicate acidity of solutions pH 7 replaces [H+ ] of 1 x 10-7 mol/litre where pH = - Log10 [H+ ] pH is temperature dependent T (°C) pH 0 7.12 10 7.06 20 7.02 25 7 30 6.99 40 6.97 pH of pure water decreases as the temperature increases A word of warning! If the pH falls as temperature increases, does this mean that water becomes more acidic at higher temperatures? NO! Remember a solution is acidic if there is an excess of hydrogen ions over hydroxide ions. In the case of pure water, there are always the same number of hydrogen ions and hydroxide ions. This means that the water is always neutral - even if its pH change Students should be able to: •define pH •describe the use of the pH scale as a measure of the degree of acidity/alkalinity •discuss the limitations of the pH scale •explain self-ionisation of water •write an expression for Kw Acid – Base Concentrations and pH concentration (moles/L) 10-1 pH = 11 pH = 3 OH- H+ pH = 7 10-7 H+ OH- OH- H+ 10-14 [H3O+] > [OH-] [H3O+] = [OH-] acidic solution neutral solution [H3O+] < [OH-] basic solution pH describes both [H+ ] and [OH- ] 0 Acidic [H+ ] = 100 pH 7 Neutral [H+ ] = 10-7 pH 14 = 0 Basic pH = 7 [H+ ] = 10-14 = 14 [OH- ] =10-14 pOH = 14 [OH- ] =10-7 pOH = 7 [OH- ] = 100 pOH = 0 pH of Common Substances Acidic Neutral Basic More acidic More basic pH NaOH, 0.1 M Household bleach Household ammonia Lime water Milk of magnesia Borax Baking soda Egg white, seawater Human blood, tears Milk Saliva Rain Black coffee Banana Tomatoes Wine Cola, vinegar Lemon juice Gastric juice 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 [H+] 1 x 10-14 1 x 10-13 1 x 10-12 1 x 10-11 1 x 10-10 1 x 10-9 1 x 10-8 1 x 10-7 1 x 10-6 1 x 10-5 1 x 10-4 1 x 10-3 1 x 10-2 1 x 10-1 1 x 100 [OH-] 1 x 10-0 1 x 10-1 1 x 10-2 1 x 10-3 1 x 10-4 1 x 10-5 1 x 10-6 1 x 10-7 1 x 10-8 1 x 10-9 1 x 10-10 1 x 10-11 1 x 10-12 1 x 10-13 1 x 10-14 pOH 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Calculations and practice • You will need to memorize the following: [H+] = 10–pH [OH–] = 10–pOH pH = – log10[H+] pOH = – log10[OH–] pH + pOH = 14 pH Calculations pH pH = -log10[H+] [H+] [H+] = 10-pH [H+] [OH-] = 1 x10-14 pH + pOH = 14 pOH pOH = -log10[OH-] [OH-] [OH-] = 10-pOH pH for Strong Acids Strong acids dissociate completely in solution Strong alkalis (bases) also dissociate completely in solution. It is easy to calculate the pH of strong acids and strong bases; you only need to know the concentration. pH Exercises a) pH of 0.02M HCl pH = – log10 [H+] = – log10 [0.020] = 1.6989 = 1.70 b) pH of 0.0050M NaOH pOH = – log10 [OH–] = – log10 [0.0050] = 2.3 pH = 14 – pOH = 14 – 2.3 =11.7 c) pH of solution where [H +] is 7.2x10-8M pH = – log10 [H+] = – log10 [7.2x10-8] = 7.14 (slightly basic) pH of dilute aqueous solutions of strong acids monoprotic e.g. HCl, HNO3 HA(aq) 0.3 M H1+(aq) + A1-(aq) 0.3 M 0.3 M pH = ? pH = - log10 [H+] pH = - log10[0.3M] pH = diprotic e.g. H2SO4 H2A(aq) 0.3 M 2 H1+(aq) + A2-(aq) 0.6 M 0.3 M 0.48 pH = - log10[H+] pH = - log10[0.6M] pH = 0.78 pH = - log [H+] Given: pH = 4.6 pH = - log10 [H+] choose proper equation 4.6 = - log10 [H+] substitute pH value in equation - 4.6 = 2nd log determine the [hydrogen ion] - 4.6 = log10[H+] antilog [H+] multiply both sides by -1 take antilog of both sides [H+] = 2.51x10-5 M 10x antilog You can check your answer by working backwards. pH = - log10[H+] pH = - log10[2.51x10-5 M] pH = 4.6 Most substances that are acidic in water are actually weak acids. Because weak acids dissociate only partially in aqueous solution, an equilibrium is formed between the acid and its ions. The ionization equilibrium is given by: HX(aq) where X- is the conjugate base. H+(aq) + X-(aq) pH calculations for Weak Acids and Weak Bases For Weak Acids pH = -Log10 For Weak Bases pOH = Log10 pH = 14 - pOH Calculating pH - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) Applying the Equilibrium Law Ka H+(aq) + A¯(aq) = [H+(aq)] [A¯(aq)] mol dm-3 (1) (2) [HA(aq)] The ions are formed in equal amounts, so therefore [H+(aq)] = Ka = [A¯(aq)] [H+(aq)]2 [HA(aq)] Rearranging (3) gives therefore [H+(aq)]2 = [H+(aq)] = [HA(aq)] Ka [HA(aq)] Ka (3) pH of solutions of weak concentrations Weak Acid pH of a 1M solution of ethanoic acid with a Ka value of 1.8 x 10-5 pH = -Log10 pH = -Log10 pH = 2.3723 pH of solutions of weak concentrations Weak Base pH of a 0.2M solution of ammonia with a Kb value of 1.8 x 10-5 pOH = -log10 pOH = -log10 pOH = 2.7319 pH = 14 – 2.7319 pH = 11.2681 Theory of Acid Base Indicators Acid-base titration indicators are quite often weak acids. For the indicator HIn The equilibrium can be simply expressed as HIn(aq, colour 1) H+(aq) + In-(aq, colour 2) The un-ionised form (HIn) is a different colour to the anionic form (In¯). Theory of Acid Base Indicators Applying Le Chatelier's equilibrium principle: Addition of acid • favours the formation of more HIn (colour 1) HIn(aq) H+(aq) + In-(aq) because an increase on the right of [H+] causes a shift to left increasing [HIn] (colour 1) to minimise 'enforced' rise in [H+]. Theory of Acid Base Indicators Applying Le Chatelier's equilibrium principle: Addition of base • favours the formation of more In- (colour 2) HIn(aq) H+(aq) + In-(aq) The increase in [OH-] causes a shift to right because the reaction H+(aq) + OH-(aq) ==> H2O(l) Reducing the [H+] on the right so more HIn ionises to replace the [H+] and so increasing In- (colour 2) to minimise 'enforced' rise in [OH-] Theory of Acid Base Indicators Summary In acidic solution HIn(aq) H+(aq) + In¯(aq) In alkaline solution Theory of Acid Base Indicators Acid-base titration indicators are also often weak bases. For the indicator MOH The equilibrium can be simply expressed as MOH(aq, colour 1) OH-(aq) + M+(aq, colour 2) Theory of Acid Base Indicators Applying Le Chatelier's equilibrium principle: Addition of base • favours the formation of more MOH (colour 1) MOH(aq) M+(aq) + OH-(aq) because an increase on the right of [OH-] causes a shift to left increasing [MOH] (colour 1) - to minimise 'enforced' rise in [OH ]. Theory of Acid Base Indicators Applying Le Chatelier's equilibrium principle: Addition of acid • favours the formation of more M+ (colour 2) MOH(aq) M+(aq) + OH-(aq) The increase in [H+] causes a shift to right because the reaction H+(aq) + OH-(aq) ==> H2O(l) Reducing the [OH-] on the right so more MOH ionises to replace the [OH-] and so increasing M+ (colour 2) to minimise 'enforced' rise in [H+] Acid Base Titration Curves Strong Acid – Strong Base Weak Acid – Strong Base Strong Acid – Weak Base Weak Acid – Weak Base Choice of Indicator for Titration Indicator must have a complete colour change in the vertical part of the pH titration curve Indicator must have a distinct colour change Indicator must have a sharp colour change Indicators for Strong Acid Strong Base Titration Both phenolphthalein and methyl orange have a complete colour change in the vertical section of the pH titration curve Indicators for Strong Acid Weak Base Titration Methyl Orange is used as indicator for this titration Only methyl orange has a complete colour change in the vertical section of the pH titration curve Phenolphthalein has not a complete colour change in the vertical section on the pH titration curve. Indicators for Weak Acid Strong Base Titration Phenolphthalein is used as indicator for this titration Only phenolphthalein has a complete colour change in the vertical section of the pH titration curve Methyl has not a complete colour change in the vertical section on the pH titration curve. Indicators for Weak Acid Weak Base Titration No indicator suitable for this titration because no vertical section Neither phenolphthalein nor methyl orange have completely change colour in the vertical section on the pH titration curve indicator pH range litmus 5-8 methyl orange 3.1 - 4.4 phenolphthalein 8.3 - 10.0 Colour Changes and pH ranges Methyl Orange Phenolphthalein Universal indicator components Indicator Low pH color Transition pH range High pH color Thymol blue (first transition) red 1.2–2.8 orange Methyl Orange red 4.4–6.2 yellow Bromothymol blue yellow 6.0–7.6 blue Thymol blue (second transition) yellow 8.0–9.6 blue colourless 8.3–10.0 purple Phenolphthalein Students should be able to: • calculate the pH of dilute aqueous solutions of strong acids and bases • distinguish between the terms weak, strong, concentrated and dilute in relation to acids and bases • calculate the pH of weak acids and bases (approximate method of calculation to be used – assuming that ionisation does not alter the total concentration of the non-ionised form) • define acid-base indicator • explain the theory of acid-base indicators • justify the selection of an indicator for acid base titrations








