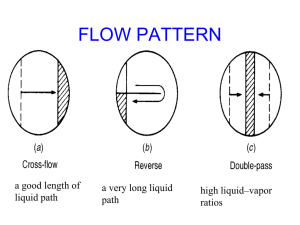
Mass Transfer Principles: Absorption, Extraction, Adsorption
advertisement
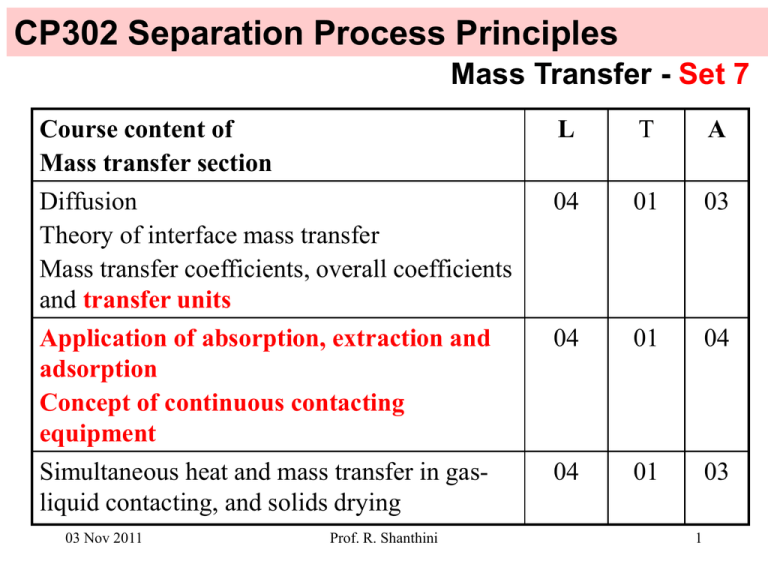
CP302 Separation Process Principles Mass Transfer - Set 7 Course content of Mass transfer section L T A Diffusion Theory of interface mass transfer Mass transfer coefficients, overall coefficients and transfer units 04 01 03 Application of absorption, extraction and adsorption Concept of continuous contacting equipment 04 01 04 Simultaneous heat and mass transfer in gasliquid contacting, and solids drying 04 01 03 03 Nov 2011 Prof. R. Shanthini 1 Example 6.10 of Ref 2 Experimental data have been obtained for air containing 1.6% by volume of SO2 being scrubbed with pure water in a packed column of 1.5 m2 in cross-sectional area and 3.5 m in packed height. Entering gas and liquid flow rates are 0.062 and 2.2 kmol/s, respectively. If the outlet mole fraction of SO2 in the gas is 0.004 and column temperature is near ambient with KSO2 = 40, calculate the following: a) The NOG for absorption of SO2 b) The HOG in meters c) The volumetric, overall mass-transfer coefficient, Kya for SO2 in kmol/m3.s 03 Nov 2011 Prof. R. Shanthini 2 Solution to Example 6.10 of Ref 2 Data: yin = 0.016 Treated gas Gout, yout Inlet solvent Lin, xin xin = 0 S = 1.5 m2 G y Z = 3.5 m L x dz G = 0.062 kmol/s G y+dy L = 2.2 kmol/s L x+dx Z z yout = 0.004 KSO2 = 40 Inlet gas Gin, yin 03 Nov 2011 Prof. R. Shanthini Spent solvent Lout, xout 3 Solution to Example 6.10 of Ref 2 Data: yin = 0.016 Operating line: xin = 0 y = (L / G) x + yout - (L / G) xin S = 1.5 m2 = (2.2/0.062) x + 0.004 Z = 3.5 m G = 0.062 kmol/s L = 2.2 kmol/s yout = 0.004 KSO2 = 40 03 Nov 2011 Equilibrium line: y=Kx = 40 x Prof. R. Shanthini 4 Solution to Example 6.10 of Ref 2 0.02 0.018 0.016 Bottom of the column (xout = ?, yin = 0.016) 0.014 y 0.012 Operating line 0.01 Equilibrium line 0.008 0.006 Top of the column (xin = 0, yout = 0.004) 0.004 0.002 0 0 0.0001 0.0002 0.0003 0.0004 0.0005 x 03 Nov 2011 Prof. R. Shanthini 5 Solution to Example 6.10 of Ref 2 a) NOG could be calculated using (83) 1 NOG = (1 - KG/L) (1 - KG/L) (yin - K xin) ln + KG/L yout - K xin where KG / L = KSO2G / L = 40*0.062 / 2.2 = 1.127 1 (1 – 1.127) (0.016 - 0) ln NOG = + 1.127 (1 – 1.127) 0.004 - 0 = 3.78 03 Nov 2011 Prof. R. Shanthini 6 Solution to Example 6.10 of Ref 2 b) HOG could be calculated using (80) HOG ≡ G KyaS = 0.062 kmol/s (Kya)(1.5 m2) Cannot continue this way since the value of Kya is not known. Use (82) instead to calculate HOG since Z and NOG are known. HOG = Z / NOG = 3.5 m / 3.78 03 Nov 2011 Prof. R. Shanthini = 0.926 m 7 Solution to Example 6.10 of Ref 2 c) Kya can be calculated using (80) HOG ≡ 0.926 m = G KyaS 0.062 kmol/s (Kya)(1.5 m2) 0.062 kmol/s Kya = (0.926 m)(1.5 m2) 03 Nov 2011 = 0.044 kmol/m3.s Prof. R. Shanthini 8 Example 6.11 of Ref 2 (modified) A gaseous reactor effluent consisting of 2 mol% ethylene oxide in an inert gas is scrubbed with water at 30oC and 20 atm. The total gas feed rate is 2500 lbmol/h, and the water rate entering the scrubber is 3500 lbmol/h. The column, with a diameter of 4 ft, is packed in two 12-ft-high sections with 1.5 in metal Pall rings. A liquid redistributer is located between the two packed sections. Under the operating conditions for the scrubber, the K-value for ethylene oxide is 0.85 and estimated values of kya and kxa are 200 lbmol/h.ft3 and 2643 lbmol/h.ft3 , respectively. Calculate the following: a) Kya b) HOG and NOG c) Yout and xout 03 Nov 2011 Prof. R. Shanthini 9 Solution to Example 6.11 of Ref 2 (modified) Treated gas Gout, yout Data: yin = 0.02 Inlet solvent Lin, xin xin = 0 G = 2500 lbmol/h G y L = 3500 lbmol/h dz S = π (4/2) ft2 = 12.6 ft2 G y+dy Z = 2 x 12 ft = 24 ft L x L x+dx Z z K = 0.85 kya = 200 lbmol/h.ft3 and kxa = 165 lbmol/h.ft3 Inlet gas Gin, yin 03 Nov 2011 Prof. R. Shanthini Spent solvent Lout, xout 10 Solution to Example 6.11 of Ref 2 (modified) a) Kya can be calculated using (78). 1 1 = + Kya kya K kxa = 1 200 + 0.85 165 Kya = 98.5 lbmol/h.ft3 b) HOG can be calculated using (80). HOG ≡ G KyaS = 2500 lbmol/h (98.5 lbmol/h.ft3)(12.6 ft2) = 2.02 ft Use (82) to calculate NOG since Z and HOG are known. NOG = Z / HOG = 24 ft / 2.02 ft = 11.88 03 Nov 2011 Prof. R. Shanthini 11 Solution to Example 6.11 of Ref 2 (modified) c) yout could be calculated using (83) 1 NOG = (1 - KG/L) (1 - KG/L) (yin - K xin) ln + KG/L yout - K xin where KG / L = 0.85 * 2500 / 3500 = 0.607 1 (1 – 0.607) (0.02 - 0) ln 11.88 = + 0.607 y 0 out (1 – 0.607) (84) yout = 0.00007 = 0.007% 03 Nov 2011 Prof. R. Shanthini 12 Solution to Example 6.11 of Ref 2 (modified) RHS of (84) Enlarge it. 18 16 14 12 10 8 6 4 2 0 0.00% 0.50% 1.00% 1.50% 2.00% 2.50% yout 03 Nov 2011 Prof. R. Shanthini 13 Solution to Example 6.11 of Ref 2 (modified) RHS of (84) yout = 0.01 mol% 18 17 16 15 14 13 12 11 10 9 8 7 6 0.00% 0.01% 0.02% 0.03% 0.04% 0.05% yout 03 Nov 2011 Prof. R. Shanthini 14 Solution to Example 6.11 of Ref 2 (modified) xout can be determined from the mass balance. xout = (G/L) yin - (G/L) yout + xin = (25/35) 0.02 - (25/35) 0.0001 + 0 = 0.0142 = 1.42% 03 Nov 2011 Prof. R. Shanthini 15 Determining the minimum liquid flow rate: Calculate (L/G)min for the removal of 90% of the ammonia from a 3540 mol/min feed gas containing 3% ammonia and 97% air. The inlet liquid is pure water and the temperature and pressure are 293 K and 1 atm, respectively. The equilibrium ratio of mole fraction of ammonia in air to mole fraction of ammonia in water at the column condition can be taken as 0.772. Since a small liquid flow rate results in a high tower (which is costly), and a large liquid flow rate requires a large diameter tower (which is also costly), the optimum liquid flow rate of 1.2 to 1.5 times the minimum flow rate is used in practice. Assuming the liquid flow rate to be 1.5 times the minimum, determine NTU, HTU and the height of tower required. Take Kya = 82 mol/m3.s and the cross-sectional area of the tower as 1.5 m 2. 03 Nov 2011 Prof. R. Shanthini 16 Solution to determining the minimum liquid flow rate: Treated gas Gout, yout Data: yin = 0.03 Inlet solvent Lin, xin xin = 0 G = 3540 mol/min G y Kammonia = 0.772 L x dz yout can be determined from the data provided as shown in the next page. G y+dy L x+dx Z z We need to calculate (L/G)min which can be done graphically as shown in the following pages. Dilute mixtures are assumed. 03 Nov 2011 Prof. R. Shanthini Inlet gas Gin, yin Spent solvent Lout, xout 17 Solution to determining the minimum liquid flow rate: Ammonia in the inlet stream = 0.03 x 3540 mol/min Air in the inlet stream = 0.97 x 3540 mol/min Ammonia removed = 0.9 x 0.03 x 3540 mol/min Ammonia in the outlet stream = 0.1 x 0.03 x 3540 mol/min Air in the outlet stream = 0.97 x 3540 mol/min yout = 0.1 x 0.03 x 3540 / (0.1 x 0.03 x 3540 + 0.97 x 3540) = 0.1 x 0.03 / (0.1 x 0.03 + 0.97) = 0.003083 ≈ 0.003 03 Nov 2011 Prof. R. Shanthini 18 Solution to determining the minimum liquid flow rate: Operating line with yout = 0.003 and xin = 0: y = (L / G) x + yout - (L / G) xin = (L / G) x + 0.003 Since L/G is not known, we cannot plot the operating line. Equilibrium line: y=Kx = 0.772 x Equilibrium line can be plotted. 03 Nov 2011 Prof. R. Shanthini 19 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (xout = ?, yin = 0.03) 0.03 y 0.025 0.02 Equilibrium line 0.015 0.01 Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 20 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (xout = ?, yin = 0.03) 0.03 y 0.025 Operating line should pass through this point. 0.02 0.015 Equilibrium line Top of the column 0.01 Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 21 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (xout = ?, yin = 0.03) 0.03 y 0.025 Operating line with the slope (L/G)min should connect these two points. 0.02 0.015 Equilibrium line 0.01 Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 22 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (xout = ?, yin = 0.03) 0.03 y 0.025 0.02 Equilibrium line 0.015 Operating line at minimum (L/G) 0.01 Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 23 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (xout = ?, yin = 0.03) 0.03 y 0.025 (L/G)min = (0.03-0.003)/xout 0.02 xout = 0.03/0.772 = 0.0389 0.015 (L/G)min = (0.03-0.003) / 0.0389 0.01 = 0.695 mol of water/mol of air Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 24 Solution to determining the minimum liquid flow rate: (L/G)min = 0.695 mol of water/mol of air G = 3540 mol/min (given) Lmin = 0.695 x 3540 = 2460 mol/min (L/G)operating = 1.5 x (L/G)min = 1.5 x 0.695 = 1.042 mol of water/mol of air Loperating = 1.042 x 3540 = 3688 mol/min 03 Nov 2011 Prof. R. Shanthini 25 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (yin = 0.03) 0.03 xout = ?? xout = 0.0389 y 0.025 0.02 Equilibrium line 0.015 Operating line at minimum (L/G) Operating line at operating (L/G) 0.01 Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 26 Solution to determining the minimum liquid flow rate: 0.04 0.035 Bottom of the column (yin = 0.03) 0.03 xout = ?? y 0.025 (L/G)operating = (0.03-0.003)/xout 0.02 Xout = (0.03-0.003)/1.042 0.015 = 0.0259 0.01 Top of the column (xin = 0, yout = 0.003) 0.005 0 0 0.01 0.02 0.03 0.04 0.05 0.06 x 03 Nov 2011 Prof. R. Shanthini 27 Solution to determining the minimum liquid flow rate: NTU (NOG) could be calculated using (83) 1 NOG = (1 - KG/L) (1 - KG/L) (yin - K xin) ln + KG/L yout - K xin where KG / L = 0.772 / 1.042 = 0.741 1 (1 – 0.741) (0.03 - 0) ln NOG = + 0.741 (1 – 0.741) 0.003 - 0 = 4.65 03 Nov 2011 Prof. R. Shanthini 28 Solution to determining the minimum liquid flow rate: HTU (HOG) could be calculated using (80) HOG = = G KyaS = 3540 mol/min (82 mol/m3.s )(1.5 m2) 3540/60 mol/s 82 x 1.5 mol/m.s = 0.48 m Height of the tower (Z) can be calculated as follows: Z = NOG x HOG = 4.65 x 0.48 m = 2.23 m 03 Nov 2011 Prof. R. Shanthini 29 Summary: Equations for Packed Columns for dilute solutions The packed height is given by: yin dy G Z = y – y* KyaS yout Treated gas Gout, yout ∫ HOG Inlet solvent Lin, xin G y dz NOG HOL G y+dy NOL L x L x+dx Z z Z = L KxaS xout dx x* – x ∫ Inlet gas Gin, yin xin 03 Nov 2011 Prof. R. Shanthini Spent solvent Lout, xout 30 Summary: Equations for Packed Columns for dilute solutions Distributed: Photocopy of Table 16.4 Alternative mass transfer coefficient groupings for gas absorption from Henley EJ and Seader JD, 1981, Equilibrium-Stage Separation Operations in Chemical Engineering, John Wiley & Sons. 03 Nov 2011 Prof. R. Shanthini 31 Gas absorption, Stripping and Extraction Gas absorption: NOG and HOG are used Stripping: NOL and HOL are used Extraction: NOL and HOL are used Humidification: NG and HG are used. 03 Nov 2011 Prof. R. Shanthini 32