Equations for Packed Columns with concentrated solutions
advertisement

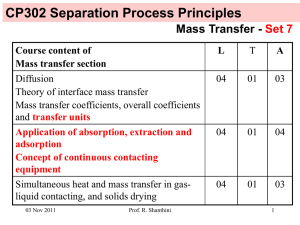
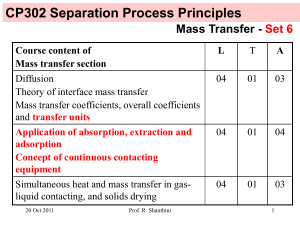
CP302 Separation Process Principles Mass Transfer - Set 8 Course content of Mass transfer section L T A Diffusion Theory of interface mass transfer Mass transfer coefficients, overall coefficients and transfer units 04 01 03 Application of absorption, extraction and adsorption Concept of continuous contacting equipment 04 01 04 Simultaneous heat and mass transfer in gasliquid contacting, and solids drying 04 01 03 24 Nov 2011 Prof. R. Shanthini 1 Treated gas Gout, yout We have learned to determine the height of packing in packed columns with dilute solutions in the last lecture class. G y Inlet gas Gin, yin Prof. R. Shanthini L x Control volume Today, we will learn to determine the height of packing in packed columns with concentrated solutions. 24 Nov 2011 Inlet solvent Lin, xin Spent solvent Lout, xout 2 Notations Gs Ls G L Y y X x - inert gas molar flow rate (constant) - solvent molar flow rate (constant) - total gas molar flow rate (varies as it looses the solute) - total liquid molar flow rate (varies as it absorbs the solute) - mole ratio of solute A in gas = moles of A / moles of inert gas - mole fraction of solute A in gas = moles of A / (moles of A + moles of inert gas) - mole ratio of solute A in liquid = moles of A / moles of solvent - mole fraction of solute A in liquid = moles of A / (moles of A + moles of solvent) Solute in the gas phase = Gs Y = G y Solute in the liquid phase = Ls X = L x 24 Nov 2011 Prof. R. Shanthini 3 Equations for Packed Columns Mass of solute lost from the gas over the differential height of packing dz = G y - G (y + dy) = - G dy Treated gas Gout, yout was used for packed column with dilute solution assuming G can be taken as a constant for dilute solutions. For concentrated solution we ought to use Gs (inert gas molar flow rate) which is constant across the column along with Y (mole ratio of solute A in gas). 24 Nov 2011 Prof. R. Shanthini Inlet solvent Lin, xin G y L x dz G y+dy L x+dx Z z Inlet gas Gin, yin Spent solvent Lout, xout 4 Equations for Packed Columns with concentrated solutions Mass of solute lost from the gas over the differential height of packing dz = Gs Y - Gs (Y + dY) = - Gs dY Treated gas Gout, yout (85) Relate Gs to G: Gs = G (1 – y) Gs Y Inlet solvent Lin, xin Ls X dz (86) Gs Y+dY Relate Y to y: Ls X+dX Z z From y = Y / (Y+1), we get Y = y / (1 – y) 24 Nov 2011 (87) Prof. R. Shanthini Inlet gas Gin, yin Spent solvent Lout, xout 5 Equations for Packed Columns with concentrated solutions Using (86) and (87), mass of solute lost from the gas over the differential height of packing dz, given by (85), can be written as follows: Treated gas Gout, yout -Gs dY = - G (1 – y) d[y / (1 – y)] = - G (1 – y) =-G dy (1 – y) dy (1 – y)2 Gs Y Ls X dz Gs Y+dY Ls X+dX Z z (88) Inlet gas Gin, yin 24 Nov 2011 Inlet solvent Lin, xin Prof. R. Shanthini Spent solvent Lout, xout 6 Equations for Packed Columns with concentrated solutions Mass of solute transferred from Treated gas Gout, yout the gas to the liquid Inlet solvent Lin, xin = Kya (y – y*) S dz where S is the inside crosssectional area of the tower. Gs Y Ls X dz Relating (88) to the above at steady state, we get -G dy (1 – y) = Kya (y – y*) S dz Compare (89) with (79) used for packed columns with dilute solutions. What are the differences? 24 Nov 2011 Prof. R. Shanthini Gs Y+dY Ls X+dX Z z (89) Inlet gas Gin, yin Spent solvent Lout, xout 7 Equations for Packed Columns with concentrated solutions Rearranging and integrating (89) gives the following: Treated gas Gout, yout Inlet solvent Lin, xin yin Z= ∫ dy G KyaS (1 – y)(y – y*) (90) Gs Y Ls X yout dz Gs Y+dY Inlet gas Gin, yin 24 Nov 2011 Prof. R. Shanthini Ls X+dX Z z Spent solvent Lout, xout 8 Equations for Packed Columns with concentrated solutions Multiply the numerator and denominator of (90) by (1 – y)LM: yin Z= ∫ (1 – y)LM dy G Kya(1 – y)LMS (1 – y)(y – y*) (91) yout where (1 – y)LM is the log mean of (1 – y) and (1 – y*) given as follows: (1 – y)LM 24 Nov 2011 y* – y = ln[(1 – y )/(1 – y* )] Prof. R. Shanthini (92) 9 Equations for Packed Columns with concentrated solutions Even though G and (1 – y)LM are not constant across the column, we can consider the ratio of the two to be a constant and take G / [Kya(1 – y)LMS] out of the integral sign in (91) without incurring errors larger than those inherent in experimental measurements of Kya. (Usually average values of G and (1 – y)LM are used.) Therefore (91) becomes the following: yin Z = K a(1G– y) S y LM ∫ (1 – y)LM dy (1 – y)(y – y*) (93) yout NOG HOG 24 Nov 2011 Prof. R. Shanthini 10 Equations for Packed Columns with concentrated solutions y* in (93) can be related to the bulk concentration using the equilibrium relationship as follows: y* = K x (94) x in (94) can be related to y in (93) using the operating line equation. We will determine the operating line equation next 24 Nov 2011 Prof. R. Shanthini 11 Equations for Packed Columns with concentrated solutions The operating equation for the Treated gas packed column is obtained by Gout, yout writing a mass balance for solute over the control volume: Lin xin + G y = L x + Gout yout Inlet solvent Lin, xin (74) If dilute solution is assumed, then Lin = L = Lout and Gin = G = Gout. G y L x Control volume We somehow have to relate L to Lin and G to Gout in (74). Inlet gas Gin, yin 24 Nov 2011 Prof. R. Shanthini Spent solvent Lout, xout 12 Equations for Packed Columns with concentrated solutions To relate L to Lin, write a mass balance for solvent over the control volume: Treated gas Gout, yout Inlet solvent Lin, xin Lin (1 – xin) = L (1 – x) L = Lin (1 – xin) / (1 – x) (95) G y To relate G to Gout, write the overall mass balance over the entire column: G + Lin = Gout + L Control volume (96) Inlet gas Gin, yin 24 Nov 2011 L x Prof. R. Shanthini Spent solvent Lout, xout 13 Equations for Packed Columns with concentrated solutions Use (95) to eliminate L from (96): G + Lin = Gout + Lin (1 – xin) / (1 – x) G = Gout + Lin (x – xin) / (1 – x) (97) Combining (74), (95) and (97), we get the following: Lin xin + [Gout+Lin(x – xin)/ (1 – x)] y = Lin(1 – xin)x/(1 – x) + Gout yout Gout yout + [Lin (1 – xin) x / (1 – x)] - Lin xin y= Gout + [Lin(x – xin) / (1 – x)] (98) Equation (98) is the operating line equation for packed columns with concentrated solutions. Compare it with equation (76) used for packed columns with dilute solutions. What are the differences? 24 Nov 2011 Prof. R. Shanthini 14 Equations for Packed Columns with concentrated solutions If the solvent fed to the column is pure then xin = 0. Therefore, (98) becomes Gout yout + [ Lin x / (1 – x) ] y= Gout + [ Lin x / (1 – x) ] 24 Nov 2011 Prof. R. Shanthini (99) 15 Example 1: Draw the operating curve for a system where 95% of the ammonia from an air stream containing 40% ammonia by volume is removed in a packed column. Solvent used in 488 lbmol/h per 100 lbmol/h of entering gas. Solution: Lin = 488 lbmol/h; Gin = 100 lbmol/h; yin = 0.4; xin = 0 In the inlet air stream: ammonia = 40 lbmol/h; air = 60 lbmol/h Ammonia removed from the air stream = 0.95 x 40 = 38 lbmol/h In the outlet air stream: ammonia = 02 lbmol/h; air = 60 lbmol/h Therefore, Gout = (60+2) = 62 lbmol/h, and yout = 2 / 62 = 0.0323 24 Nov 2011 Prof. R. Shanthini 16 Example 1: Using Lin = 488 lbmol/h, Gout = 62 lbmol/h, yout = 0.0323 and xin = 0 in (98), we get the operating curve as follows: y 62 x 0.0323 + [488 x / (1 – x)] y= 62 + [488 x / (1 – x)] 0.5 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 yin (bottom of the tower) Operating curve 0 0.02 0.04 0.06 0.08 0.1 yout (top of the tower) x 24 Nov 2011 Prof. R. Shanthini 17 Example 2: Draw the equilibrium curve, which is approximately described by K = 44.223x + 0.4771, on the same plot as in Example 1. y Solution: 0.5 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 Operating curve Equilibrium curve y=Kx = 44.223x2 + 0.4771x 0 0.02 0.04 0.06 0.08 0.1 x 24 Nov 2011 Prof. R. Shanthini 18 Example 3: Determine the NOG using the data given in Examples 1 and 2. Solution: yin NOG = ∫ (1 – y)LM dy (1 – y)(y – y*) yout where (1 – y)LM y* – y = ln[(1 – y )/(1 – y* )] Given x, calculate y from the operating curve and y* from the equilibrium curve. Using those values, determine the integral above that gives NOG. 24 Nov 2011 Prof. R. Shanthini 19 Example 3: The shaded area gives NOG as 3.44 (Numerical integration is better suited to get the answer.) 35 Integrand 30 25 20 15 10 5 0 0 0.1 yout = 0.0323 24 Nov 2011 0.2 y Prof. R. Shanthini 0.3 0.4 yin = 0.4 20 Example 4: Determine the height of packing Z using the data given in Examples 1 and 2. Solution: HOG = G Kya(1 – y)LMS Z = NOG HOG 24 Nov 2011 Need more data to work it out. Can be calculated once HOG is known. Prof. R. Shanthini 21 Summary with overall gas-phase transfer coefficients for packed column with concentrated solutions yin G Z = K a(1 – y) S y LM ∫ (1 – y)LM dy (1 – y)(y – y*) (93) yout NOG HOG where (1 – y)LM is the log mean of (1 – y) and (1 – y*) given as follows: y* – y (1 – y)LM = (92) ln[(1 – y )/(1 – y* )] 24 Nov 2011 Prof. R. Shanthini 22 Summary with overall liquid-phase transfer coefficients for packed column with concentrated solutions L Z = K a(1 – x) S x LM xout (1 – x)LM dy (1 – x)(x* – x) ∫ (93) xin NOL HOL where (1 – x)LM is the log mean of (1 – x) and (1 – x*) given as follows: x* – x (1 – x)LM = (92) ln[(1 – x )/(1 – x* )] 24 Nov 2011 Prof. R. Shanthini 23 Summary: Equations for Packed Columns for dilute solutions Distributed already: Photocopy of Table 16.4 Alternative mass transfer coefficient groupings for gas absorption from Henley EJ and Seader JD, 1981, Equilibrium-Stage Separation Operations in Chemical Engineering, John Wiley & Sons. 24 Nov 2011 Prof. R. Shanthini 24 Gas absorption, Stripping and Extraction Gas absorption: NOG and HOG are used Stripping: NOL and HOL are used Extraction: NOL and HOL are used Humidification: NG and HG are used. 24 Nov 2011 Prof. R. Shanthini 25