Electrostatics PowerPoint
advertisement

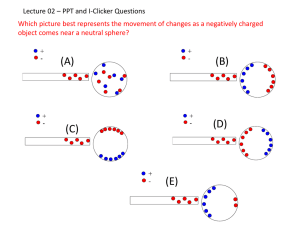
Electric Forces and Fields Static electricity is the accumulation of electrical charges on the surface of a material, usually an insulator or non-conductor of electricity. It is called “static” because there is no current flowing 1 Typically, two materials are involved in static electricity, with one having an excess of electrons or negative (−) charges on its surface and the other material having an excess of positive (+) electrical charges. Atoms near the surface of a material that have lost one or more electrons will have a positive (+) electrical charge. 2,3 As the neutrally charged person walks across the wool carpet, his leather soled shoes have less desire for electrons than the wool carpet. As a result, electrons get stolen from the shoe by the carpet. With every step the person becomes more and more positively charged. That charge distributes itself over the body. When the positively charged person gets near the metal door he will actually attract charges from the door which jump in the form of a spark. Notice how only the negative charges (electrons) are free to move. The process causes electrons to be pulled from the surface of one material and relocated on the surface of the other material. It is called the triboelectric effect or triboelectric charging. The material that loses electrons ends up with an excess of positive (+) charges. The material that gains electrons ends up an excess of negative (−) charges on its surface. 4,5 A substance that is lower on the list TAKES electrons (becomes negatively charged) from a substance that is higher on the series. 7 If two items from the list are rubbed together, then the item that is higher on the list will end up more positive and the lower one will end up more negatively charged. For example, if leather were rubbed with wool, the leather becomes positive and the wool negative. Yet if rubber is rubbed with wool, the rubber becomes negative and the wool positive. It is important to note that this series is true only if the samples are clean and dry. The presence of moisture, dirt, or oils may cause some of the items to interact differently. 8 Rub a balloon filled with air on a wool sweater or on your hair. Then hold it up to a wall. The balloon will stay there by itself. When you rub the balloon it picks up extra electrons from the sweater or your hair and becomes slightly negatively charged. The negative charges in the single balloon are attracted to the positive charges in the wall. Tie strings to the ends of two balloons. Now rub the two balloons together, hold them by strings at the end and put them next to each other. They'll move apart. Rubbing the balloons gives them static electricity. The two balloons hanging by strings both have negative charges. There are two kinds of electric charge: Positive and negative. A basic law of the universe is that like charges repel and unlike charges attract. A negative and a positive charge will attract each other. Two positive charges or two negative charges will repel each other. 9 Electric charge is conserved. If you rub a balloon on your hair, some of your hair’s electrons are transferred to the balloon. The balloon gains a certain amount of electrical charge while your hair loses an equal amount of negative charge. 10 The positive charge on your hair is equal in magnitude to the negative charge on the balloon. Charges can be transferred from one object to another; however, electric charge is conserved in this process; no charge is created or destroyed. 11 Electric charge is quantized. In 1909, Robert Millikan (1886 – 1953) performed his famous oil drop experiment at the University of Chicago in which he observed the motion of tiny oil droplets between two parallel metal plates. After repeating this process for thousands of drops, Millikan found that when an object is charged, its charge is always a multiple of a fundamental unit of charge, symbolized by the letter e. 12, 13, 14 Other experiments by Millikan’s time demonstrated that the electron has a charge of –e and the proton has a charge of +e. The value of e (a single electrical charge unit) has since been determined to be 1.60219 x 10-19 C. 15, 16, 17 The Coulomb (C) is the SI unit of charge. Materials in which electric charge moves freely, such as copper and aluminum (most metals) are called conductors. The animation is showing a neutrally charged conductor and its response to charged objects being brought near it. 18 As the positively charged rod is brought near the conductor, the electrons are attracted toward the charged rod. This causes a force of attraction to be created between the rod and the conductor. As the negatively charged rod is brought near the conductor, the electrons are repelled away from the charged rod. This causes a force of attraction to be created between the rod and the conductor. Notice that only the electrons are free to move. It is also important to notice that when no charged object is near the conductor, the electrons evenly distribute themselves within the conductor. Charging a conductor by induction. Induction is the process of charging a conductor by bringing it near another charged object and grounding the conductor. Charged object does not touch the electroscope. Electroscope ends up oppositely charged to the object used to charge it. The first charge is strong and stays strong each time the electroscope is recharged. (This is due to the original object not losing any charge in the process.) 19 Conduction or Induction A B Induction Polarization Materials in which electric charges do not move freely , such as glass, rubber, silk and plastic are called insulators. 20 This animation is showing a neutrally charged insulator and its response to a charged object being brought near it. In an insulator (such as plastic, rubber, glass, etc) the electrons are not free to move around the entire object. They are generally restricted to moving only around the atom they are attached to. They can move from one side of the atom to the other but are unable to leave the atom. As a result, we say that charges stay where you put them on an insulator. 21,22 Inducing surface charge on insulators by polarization In the animation, the electrons are evenly distributed but are attached to only one of the positive charges. As the negatively charged rod is brought near the insulator, the electrons move to the other side of the positive charges but are unable to move completely to the far side of the object. This results in polarization where more positive charges are on one side of the molecule than on the other. You should notice that the upper side of the insulator becomes more positive and feels a force of attraction to the the charged object. This would also be true if the object was positively charged. Therefore, a neutral insulator will always be attracted 23,2 to a charged object. Semiconductors are a third class of materials characterized by electrical properties that are somewhere between those of insulators and conductors. In their pure state, semiconductors are insulators. But the carefully controlled addition of specific atoms as impurities can dramatically increase a semiconductor’s ability to conduct electric charge. 25, 26, 27 Silicon and Germanium are two well-known semiconductors that are used in a variety of electronic devices. 28 Superconductors become perfect conductors when they are at or below a certain temperature. 29 Insulators and conductors can be charged when objects come into direct contact with each other. this process is known as charging by contact. Examples of charging by contact: Rubbing a balloon in your hair. Rubbing a glass rod with silk. The two rods become oppositely charged and attract one another. 30, 31 Conductors can be charged by induction. Induction is the process of charging a conductor by bringing it near another charged object and grounding the conductor. When a conductor is connected to Earth by means of a conducting wire or copper pipe, the conductor is said to be grounded. The Earth can be considered an infinite reservoir for electrons because it can accept or supply an unlimited number of electrons. 32, 33, 34 Michael Faraday made his discovery of electromagnetic induction in 1831, he hypothesized that a changing magnetic field is necessary to induce a current in a nearby circuit. To test his hypothesis he made a coil by wrapping a paper cylinder with wire. He connected the coil to a galvanometer, and then moved a magnet back and forth inside the cylinder. Faraday confirmed that a moving magnetic field is necessary in order for electromagnetic induction to occur. 35, 36 Charles Coulomb established The inverse square law for electric force between two charges. He used a torsion balance like the one shown here in his experiment. Coulomb’s experiment showed that electric repulsion obeys a law having the same form as Newton’s law of gravity. The device, a torsion balance, measured extraordinarily small forces, relying on a single filament of silk suspended from a pure silver wire thin as a hair. 38, 39 Because like charges repel, the spheres on the torsion balance twist away from the other spheres. By knowing the distance between the spheres, the force needed to twist them (the torque from which the torsion balance gets it name), and the charges on the spheres, Coulomb could figure out a formula. Coulomb's law states that The electrical force between two charged objects is directly proportional to the product of the quantity of charge on the objects and inversely proportional to the square of the separation distance between the two objects. In equation form, Coulomb's law can be stated as 40, 41 FE represents the electrical force between two objects. Q1 represents the quantity of charge on object 1 (in Coulombs) Q2 represents the quantity of charge on object 2 (in Coulombs) d represents the distance of separation between the two objects (in meters). k is a proportionality constant known as the Coulomb's law constant. The value of this constant is dependent upon the medium that the charged objects are immersed in. In the case of air, the value is approximately 8.99 x 109 N • m2 / C2. 42 Electric Fields A Van de Graaff generator is an Electrostatic generator which uses a moving belt to accumulate very high electrostatically stable voltages on a hollow metal globe on the top of the stand. A pulley drives an insulating belt by a sharply pointed metal comb which has been given a positive charge by a power supply. Electrons are removed from the belt, leaving it positively charged. A similar comb at the top allows the net positive charge* to spread to the dome. When you approach a Van de Graaf generator, you can sense the electrical charge surrounding the dome. The hair on your arms stand up, just a tiny bit if you are more than a meter away and more if you are closer. It is obvious that the space surrounding the dome is altered somehow by the electric charges. The space is said to contain an electric field. A charged object sets up an electric field in the space around it. For example, an electric force holds an electron in orbit around a proton. In this case, there is no contact between the objects and the forces are acting “at a distance”. Here, the electron is interacting within the electric field of the proton. The force that one electric charge exerts on another can be described as the interaction between on charge and the electric field set up by another. An electric field is a region in space around a charged object in which a stationary charged object experiences an electric force because of its charge. Electric field is a vector quantity and has both magnitude and direction.can be represented by a vector arrow. Electric field strength depends on charge and distance. For any given location, the arrows point in the direction of the electric field and their length is proportional to the strength of the electric field at that location. Such vector arrows are shown in the diagram below. Note that the length of the arrows are longer when closer to the source charge and shorter when further from the source charge. Electrical Field • Michael Faraday (1791 – 1867) developed an approach to discussing fields • An electric field is said to exist in the region of space around a charged object – When another charged object enters this electric field, the field exerts a force on the second charged object Electric Field Strength The only way we can tell if a field exists is to place a test charge at that spot and see if it feels a force. (In other words, it takes one to know one.) When a test charge is brought in, a force is present on that charge and so it shows evidence of a field being present. The closer the test charge is brought to the stationary charge the greater the force. The greater the force on the test charge, the stronger the field is. Electric Field Strength Field Strength is described as the ratio of Force to the amount of charge. The field intensity for an electric field is measured in Newtons per Coulomb [N/C]. This describes the amount of force present for every coulomb of charge used as a test charge. The field strength equation (on the next slide) has no way of specifying the direction of the field, therefore you should ignore any negative signs that get created in your answer. Electric Field • A charged particle, with charge Q, produces an electric field in the region of space around it • A small test charge, qo, placed in the field, will experience a force Electric Field Definition ke Q F • Mathematically, E 2 qo r • SI units are N / C • The electric field is a vector quantity • The direction of the field is defined to be the direction of the electric force that would be exerted on a small positive test charge placed at that point. Electric Field due to a Positive Spherical or Point Charge • The electric field produced by a positive charge is directed away from the charge – A positive test charge would be repelled from the positive source charge • The magnitude of the electric field produced by the positive charge is More About a Test Charge and The Electric Field • The test charge is required to be a small charge – It can cause no rearrangement of the charges on the source charge • The electric field exists whether or not there is a test charge present Electric Field due to a Negative Spherical or Point Charge • The electric field produced by a negative charge is directed toward the charge – A positive test charge would be attracted to the negative source charge • The magnitude of the electric field produced by the negative charge is A charged object sets up an electric field around it. You can show electric field patterns using: Electric Field Lines The concept of electric field lines was introduced by Michael Faraday. Although electric field lines don’t really exist, they offer a useful means of analyzing fields. Electric field lines reveal information about the direction and the strength of an electric field within a region of space. This is useful because the field at each point is often the result of more than one charge. If the charge that sets up the field is positive, the field points away from the charge. If the charge that sets up the field is negative, the field points toward the charge. For a single isolated charge, the lines extend to infinity. Electric Field Lines Rules for Drawing Electric Field Lines 1. The lines must originate on a positive charge (or infinity) and end on a negative charge (or infinity). 2. The number of lines drawn leaving a positive charge or approaching a negative charge is proportional to the magnitude of the charge. 3. No two field lines can cross each other. 4. The line must be perpendicular to the surface of the charge Where the electric field lines are farther apart, the field is weaker. The higher the density of the electric field lines around a charge, the greater the quantity of charge will be. The three objects above reveal, that the quantity of Charge on C is greater than the quantity of charge on B which is greater than the quantity of charge on A. • An electric dipole consists of two equal and opposite charges. • The high density of lines between the charges indicates the strong electric field in this region. Electric Field Line Patterns Electric Field Line Patterns • Two equal but like point charges • The bulging out of the field lines between the charges indicates the repulsion between the charges • The low field lines between the charges indicates a weak field in this region Electric Field Patterns • Unequal and unlike charges • Note that two lines leave the +2q charge for each line that terminates on -q The number of electric field lines is proportional to the electric field strength. Conductors in Electrostatic Equilibrium • When no net motion of charge occurs within a conductor, the conductor is said to be in electrostatic equilibrium • An isolated conductor in electrostatic equilibrium has four important properties Conductors – Property 1 When a conductor is in electrostatic equilibrium, only points on the surface have net charge. The electric field is zero everywhere inside the conducting material. Property 1 • The electric field is zero everywhere inside the conducting material – Consider if this were not true: • If there were an electric field inside the conductor, the free charge there would move and there would be a flow of charge • If there were a movement of charge, the conductor would not be in equilibrium Conductors – Property 2 When a conductor is in electrostatic equilibrium, the charges are concentrated at regions of greater curvature. Property 2 • On an irregularly shaped conductor, the charge accumulates at locations where the radius of curvature of the surface is smallest (that is, at sharp points) • Why a lightning rod works Conductors – Property 3 When a conductor is in electrostatic equilibrium, the Electric field at the surface is perpendicular. • The electric field just outside a charged conductor is perpendicular to the conductor’s surface Consider what would happen if it this was not true – The component along the surface would cause the charge to move – It would not be in equilibrium Property 4 • Any excess charge on an isolated conductor resides entirely on its surface – A direct result of the 1/r2 repulsion between like charges in Coulomb’s Law – If some excess of charge could be placed inside the conductor, the repulsive forces would push them as far apart as possible, causing them to migrate to the surface Experiment to Verify Properties of Charges Michael Faraday used a metal ice pail as a conducting object to study how charges distribute themselves when a charged object was placed inside the pail. • Faraday’s Ice-Pail Experiment – A charged object suspended inside a metal container causes a rearrangement of charge on the container so that the sign of the charge on the inside surface of the container is opposite the sign of the charge on the suspended object – Any charge transferred to a conductor resides on its surface in electrostatic equilibrium Electrostatic spray painting (Powder-coating) The atomized particles are made to be electrically charged, thereby repelling each other and spreading themselves evenly as they exit the spray nozzle. When a spray gun is charged, the electric field around the sharp points can be strong enough to produce a corona around the gun. Air molecules in this area are ionized and paint droplets pick up negative charges from these molecules as they pass through the ionized air. This method also means that paint covers hard to reach areas. Car body panels and bike frames are two examples where electrostatic spray painting is often used. Electrosatic Spray Painting The End