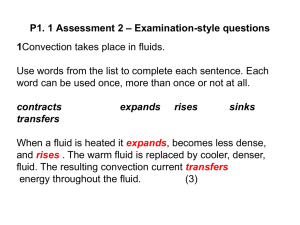
q. ndA
advertisement
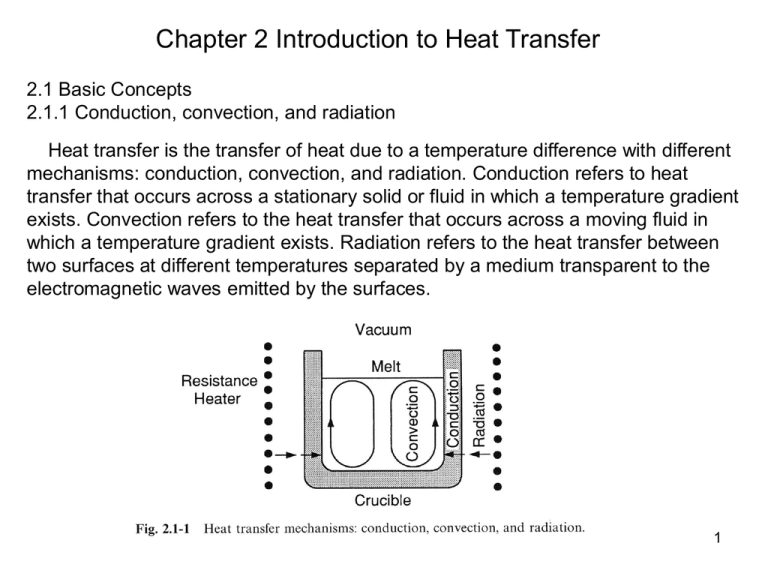
Chapter 2 Introduction to Heat Transfer 2.1 Basic Concepts 2.1.1 Conduction, convection, and radiation Heat transfer is the transfer of heat due to a temperature difference with different mechanisms: conduction, convection, and radiation. Conduction refers to heat transfer that occurs across a stationary solid or fluid in which a temperature gradient exists. Convection refers to the heat transfer that occurs across a moving fluid in which a temperature gradient exists. Radiation refers to the heat transfer between two surfaces at different temperatures separated by a medium transparent to the electromagnetic waves emitted by the surfaces. 1 2.1.2 Fourier’s law of conduction 2.1.2.1 One-dimensional Consider the conduction of heat through a slab of thickness L, as shown in Fig. 2.1-2. The lower and upper surfaces are kept at a constant temperature T1 and T2, respectively. A steady-state temperature profile T(y) is established in the slab. Consider two surface in slab separated with a infinitesimal distance dy, as shown in Fig. 2.1-2. due to temperature gradient generated in the slab, heat flow from the surface y to the surface y+dy. A heat flux is defined as the amount of heat transferred per unit area per unit time, and can be expressed as dT q y k dy [2.1-1] where k is the thermal conductivity of the medium. This equation is Fourier’s law of conduction for one-dimensional heat conduction in the y-direction. The mks units of the heat flux and the thermal conductivity are W/m2 and Wm-1K-1, respectively. 2 The thermal conductivities of some common materials are given in Fig. 2.1-3 and Table 2.1-1. 2.1.2.2 Three-dimensional For heat transfer in a three-dimensional medium, the Fourier’s law can be expressed for each of the three coordinate directions q x k dT [2.1-2] dx dT q y k [2.1-3] dy dT q z k [2.1-4] dz And can be expressed in a three-dimensional form of Fourier’s law of conduction. q kT 2.1.2.3 The Thermal Diffusivity The thermal diffusivity, α , is defined as k Cv Where and Cv are the density and specific heat of the material, respectively. 3 2.1.3 Thermal boundary layer Consider a fluid of uniform temperature T∞ approaching a flat plate of constant temperature Ts in the direction parallel to the plate. At the solid/liquid interface the fluid temperature is Ts since the local fluid particles achieve thermal equilibrium at the interface. The fluid temperature T in the region near the plate is affected by the plate, varying from Ts at the surface to T∞ in the main stream. This region is called the thermal boundary layer. 4 Definition of thermal thickness: The thickness of thermal boundary layer δT is taken as the distance from the plate surface at which the dimensionless temperature (T-TS)/(T∞-TS) reaches 0.99. In practice it is usually specified that T=T∞ and T / y 0 at y=δT. The effect of conduction is significant only in the boundary layer. Beyond it the temperature is uniform and the effect of conduction is no longer significant. A fluid of uniform temperature T entering a circular tube of inner diameter D and uniform wall temperature TS, as illustrated in Fig. 2.1-5. A thermal boundary layer begins to develop at the entrance , gradually expanding until the layers from oppositive sides approach the centerline. This occurs at v D C z 0.05 v 0.05Re D Pr D k where v D Re D Inertial force Viscous force (Reynolds number) Cv Pr k Cv (Prandtl number) [2.1-7] Viscous diffusivity [2.1-8] thermal diffusivity 5 Define average temperature Tav C TvdA C vdA A A v [2.1-10] v The thermally fully developed temperature profile in a tube is one with a dimensionless temperature (Ts-T)/(Ts-Tav) independent of the axial position, that is TS T 0 z TS Tav [2.1-12] 2.1.4 Heat transfer coefficient Consider the thermal boundary layer. At the solid/liquid interface heat transfer occurs only by conduction since there is no fluid motion. Therefore, the heat flux across the solid/liquid interface is T q y y 0 k [2.1-13] y 0 y This equation cannot be used to calculate the heat flux when the temperature gradient is an unknown. A convenient way to avoid this program is to introduce a heat transfer coefficient, defined as follows: q y y 0 k T y y 0 [2.1-14] h Ts T Ts T 6 The absolute values are used to keep h always positive. From Eq. [2.1-14] qy y 0 h (TS T ) [2.1-15] The equation is called Newton’s law of cooling. For fluid flow through a tube of an inner radius R and wall temperature TS, a similar equation can be used: h qr r R TS Tav k T r TS Tav r R [2.1-16] Where Tav is the average fluid temperature over the cross-sectional area pR2. Consider the thermally fully developed region shown in Fig. 2.1-5. In the case of a constant heat flux, the heat transfer coefficient h is constant in the thermally fully developed region. From Eq. [2.1-16] we see that (TS-Tav) is also constant. From this and Eq.[2.1-12], we have Ts T Tav z z z (constant qr r R ) [2.1-17] Since TS and Tav are independent of r, T / zis also independent of r, Let us consider the case of a constant wall temperature TS. Eq. [2.1-12] can be expanded and solved for T / z to give 7 T T Tav T ( S ) z TS Tav z (constant TS) [2.1-18] Since T is dependent on r, T / z is also dependent on r. 2.2 Overall energy-balance equation 2.2.1 Derivation Consider a control volume Ω bounded by control surface A through which a moving fluid is flowing. As defined in previous chapter, the control surface is composed by Ain , Aout, and Awall. Consider an infinitesimal area dA in vector form is ndA, the inward and outward heat transfer rate through area dA is -q. ndA and q. ndA, respectively. The energy conservation law (first law of thermodynamics) written for an open system under unsteady-state condition is rateof energy rateof energyin rateof energyout (1) (2) (3) accumulation by mass inflow by mass outflow rateof otherheat rateof work rateof heat [2.2-3] transfer ot system (4) done by system (5) generation (6) fromsurroundings on surroundings in system 8 Term 1: Rate of energy accumulation The thermal, kinetic, and potential energy per unit mass of the fluid are CvT, v2/2, and ψ, respectively, where Cv, T, v are the specific heat, temperature, and velocity of the fluid. The total energy per unit mass of the fluid 1 2 et CvT v 2 [2.2-4] The total energy in the differential volume element dΩis dEt=ρetdΩ. dm =ρdΩ This can be integrated over Ω to obtain the total energy in the control volume Et et d (integral) dEt et d And the rate of energy change in Ω is dt t Et (overall) = Terms 2 & 3: Rate of energy in by mass inflow The inward energy flow rate is energy per unit mass, et, times inward mass flow rate through dA and can be expressed as et v ndA A Since v=0 at the wall, above term can be expressed as ( et v ndA)in ( et v ndA) out (0) wall A A 9 Term 4: Rate of heat transfer The heat transfer by conduction dA is Q q ndA A Term 5: The rate of work done by the fluid in the C.V. on the surroundings, includes: (1) The rate of shaft work done The rate of shaft work done by the fluid in the C.V. on the surroundings, that is, through a turbine or compressor, is Ws (2) The rate of pressure work done To leave the C.V. through dA, the fluid has to work against the pressure of the surrounding fluid. Since the pressure force is pndA, the rate of pressure work required is dWp= pv‧ndA. Therefore, the rate of pressure work the fluid has to do to go through the C.V. is W p pv ndA A (3) The rate of viscous work done To overcome the viscous force t‧ndA, the rate of viscous work required is dWv= (t‧n)‧vdA. The rate of viscous work the fluid has to do is Wv (t v) ndA A 10 Term 6: Heat generation rate The heat generation rate per unit volume, such as that due to Joule heating, phase transformation, or chemical reaction. The rate of heat generation in the differential volume element dΩ is dS=s dΩ. The heat generation in the control volume is S, S sd Substituting the integral form of terms(1) through (6) into Eq.[2.2.3] et d t = et v ndA q ndA A A [2.2-5] pv ndA (t v) ndA W s A A In most problem, including those in materials processing, the kinetic and potential energies are neglegible as compared to the thermal energy. Furthermore, the pressure, viscous and shaft work are usually negligible or even absent. As such, Eq.[2.2-5] reduces to CvTd CvTv ndA q ndA sd A A t [2.2-6] 11 According to [2.2-3], Eq. [2.2-6] can be written as ET ( CvTvdA)in ( CvTvdA) out Q S A A t [2.2-7] Where ET is the thermal energy in the control volume, substituting Eq. [2.1-11] (definition of Tav) into this equation and assuming constant Cv, we obtain ET ( CvTav vav A)in ( CvTav vav A) out Q S t (m CvTav ) in (m CvTav ) out Q S [2.2-8] 2.2.2 Bernoulli’s Equation Consider the steady-state isothermal flow of an inviscid incompressible fluid without heat generation, heat conduction, shaft work, and viscous work. Substituting Eq. [2.2-4] into [2.2-5] and assuming uniform properties over the cross-sectional area A, we have 12 0 (et A p ) v ndA 1 p 1 p (Cv T v 2 )vA (Cv T v 2 )vA 2 2 1 2 [2.2-9] Since T1=T2 and (vA)1=(vA)2, Eq.[2.2-9] reduces to 1 2 p1 1 2 p2 v1 1 v 2 2 2 2 If the z direction is taken vertically upward, =gz, where g is the gravitational acceleration. As such, Eq/.[2.2-10], on multiplying by , becomes 1 1 v12 gz1 p1 v 2 2 gz 2 p2 2 2 or simply 1 2 v gz p constant 2 Which is the Bernoulli equation. 13 Example 2.2.2 Conduction through cylindrical composite wall 14 Example 2.2.3 Heat transfer in fluid flow through a pipe Based on the C.V. selected in the figure mCvTav mCv (Tav dTav ) dQ 0 dQ mCv dTav dQ Based on the definition of overall Heat transfer coefficient dQ 2p RdzU (T Tav ) (A) B.C2: T TL at z=L ln mCv dTav 2p RdzU (T Tav ) d (T Tav ) 2p RU dz (T Tav ) mCv 2p RU ln(T Tav ) z C mCv B.C.1: Tav Tz at z=0 C=ln(T T0 ) ln (T Tav ) 2p RU z (T T0 ) mCv T TL (T T0 ) e - (T TL ) 2p RU L (T T0 ) mCv 2p RU L mCv (T T0 ) (TL T0 ) (T T0 ) e TL T0 (T T0 ) (1-e - 2p RU L mCv - 2p RU L mCv ) 15 Example 2.2.4 Counterflow heat exchanger Given: Hot stream Th1, Th2, mh cold stream inlet(Tc2), outlet(Tc1), and mass flow rate mc Overall heat transfer coefficient U, turbulent Find Qe (steady state heat exchange rate Qe in terms of U, Th and Tc). For hot stream 0 mhCvTh1 mhCvTh2 (Qe ) From view For cold stream 0 mcCvTc2 mcCvTc1 Qe of Th2 Th1 1 overall Therefore [2.2-47] m C Q h v e and Tc2 Tc1 1 [2.2-48] mcCv Qe 16 From view of C.V. Because we want to express Qe in terms of U, therefore, considering the C.V. in the inner pipe. 0 mhCvTh mhCv (Th dTh ) [(2p RdzU )(Th Tc )] energy in energy out dTh 2p RUdz Th Tc mhCv heat loss from the wall [2.2-50] Similarly, for the C.V. in the outer pipe 0 mhCv (Tc dTc ) mcCvTc (2p RdzU )(Th Tc ) energy in and energy out dTc 2p RUdz Th Tc mcCv gain heat the wall [2.2-52] Subtracting Eq.[2.2-52] from Eq. [2.2-50], we have d (Th Tc ) 1 1 2p RU ( )dz [2.2-53] Th Tc mhCv mcCv 17 Integrating from z=0 to z=, and substituting [2.2-47] and [2.2-48] to [2.2-53] Th 2 Tc 2 Th1 Tc1 or d ln(Th Tc ) L 2p RU (Th 2 Th1 Tc 2 Tc1 ) dz 0 Qe (2.2-54) (T T ) (T T ) h1 c1 (2.2 55) Qe (2p RL)U h 2 c 2 (T T ) ln h 2 c 2 (Th1 Tc1 ) 18 Example 2.2.5 Heat transfer in laminar flow over a flat plate Given: Steady state, constant physical properties, no heat generation Find: dT and heat transfer coefficient Approach: 1. Construct C.V. 2. Find the mass flow rate into the C.V. from surface 4 d dT m4 [ vz dy ]dz dz 0 3. Consider the energy balance dT 0 dT CvT vz dy z CvT vz dy z z qy 0 y 0 z m4CvT 0 4. Substituting m4 into Eq. [2.2-57], and according to Fourier’s law of conduction qy y 0 T k y y 0 19 we have dT 0 CvT vz dy z dT 0 T CvT vz dy z z k y dividing by Cv d dT T [ Tvz dy]dz dz 0 y T y y 0 d dT z ( CvT vz dy )z 0 dz 0 y 0 d dT z ( Tvz dy )dz 0 dz 0 y 0 d dT vz (T T )dy dz 0 5. Assume and T Ts 3 y 1 y 3 = ( ) - ( ) [2.2-61] T Ts 2 d T 2 dT vz 3 y 1 y = ( ) - ( )3 [2.2-64] v 2 d 2 d 6. Assume Please see the derivation in other pages 1 Cv dT 3 dT d , we have Pr , [2.2-65] where Pr= d k 20 7. By the definition of heat transfer coefficient k h T y y 0 Ts T we have h = [2.2-67], substituting Eq[2.2-61] into [2.2-67] 3 k [2.2 68] 2 dT From Eq.[1.4-62] we have z d = 4.64 [2.2 69] Rez From Eqs. [2.2-65], [2.2-68], and [2.2-69] We have 1 1 1 hz 3 z 3 d z 3 13 1 =Nu z Pr Re z 2 0.323Pr 3 Re z 2 k 2 dT 2 dT d 2 4.64 [2.2-65] [2.2-69] 21