High Resolution Structure of the Open NaK Channel
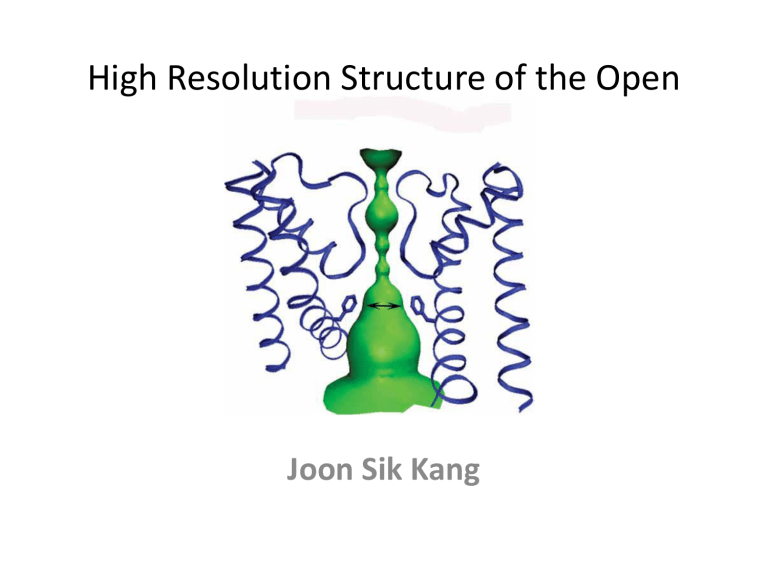
High Resolution Structure of the Open
NaK Channel
Joon Sik Kang
Background
• Opening & Closing of ion channel pores in response to ligand or voltage is the fundamental studies of channel physiology.
• Most of the studies have been from the K+ channels of which KcsA(closed) and MthK(open) have been accepted.
• Closed Conformation: Straight line helices and bundle crossing
• Open Conformation: Inner helices bent at glycine residue(gating hinge)
Objective/Method
• Nak Channel chosen, because it shares overall sequence and structural similarities with KcsA.
• Crystallized the truncated construct of
NaK(NaKND19), which lacks N-terminal M0 helix, and viewed at 1.6 A Resolution.
• Crystallized in the presence of monovalent cations.
• Unexpectidly, NaKND19 revealed intracellular gate in an open conformation.
Result
• <Overall Structure of Open NaK>
• <Conservation of Pore Opening Mechanism>
• <Inter&Intrasubunit Interaction in
Closed,Open Pore>
• <Effect on Ion-Conduction Pathway Size on
Channel Conduction>
Overall NaK Structure
Overall NaK Structure
• 2 subunits, each use 3 four-fold counterparts to form a functional channel tetramer.
• Region surrounding selectivity filter are similar for all channels, suggesting that this region remain static during channel gating.
• Major Structure change occur in the pore lining inner-helices just below the selectivity filter.
Overall NaK Structure
• Inner helices of NaK straight, as those of KcsA whereas NaKND19 develops bend (1D), occuring at Gly 87 in NaK.
• Inner helices undergo bending and twisting around the helical axis.
Conservation of Pore Opening
Mechanism
• In the closed conformation, inner and pore helices superimpose well, but outer helices are slightly shifted translationally.
• The fact that all 4 of these have similarities in structure indicate that there is conserved gating mechanics on tetrameric cation channel.
• But. Outer helices do not need same level of conservation.
Inter&Intrasubunit interaction in closed/Open Pore
Inter&Intrasubunit interaction in closed/Open Pore
• The intrasubunit interaction between inner&outer helices remain similar between closed and open state, because of the concurrent movement of both helices and twisting.
• However, interaction is disrupted upon pore opening (zoom 3).
Continued
• In Closed conformation, Phe 92 from each inner helix form contact with neighboring Phe
92. (Zoom 2A)
• Inner helix bending in open conformation causes Phe 92 to swing away and point its side chain toward the central ion-conducting pathway.
Effect Of ion-conduction pathway size on channel conduction
• In a closed NaK channel, side chain resides around bundle crossing form constriction points along the ion-conduction pathway.
• Bundle of inner helices in open form, disrupts the bundle crossing.
• However a new point is created through the
Phe 92.
Continued
• In most cases, Positions of Phe 92 is occupied by smaller residues such as alanine.
• Positioning its aromatic ring toward the ion pathway, Phe-92 form constriction point about size of 6.5 A in diameter. However, MthK, being occupied by smaller alanines, have larger diameter, allowing higher conduction rate.
Effect of Ion-Conduction Pathway size on channel conduction
Continued
• F92A mutant shows an pathway that is less hindered with the diameter of 10.5 A
• Smaller the side chain residue, wider the ion passageway, thus easier access to the selectivity filter in the cation channels.
Discussion
• The region surrounding the selectivity filter remain static during the channel gating.
• However, inner helices at Gly 87, just below the filter, show major conformational change.
• NaKND19 and MthK similar in the use of
Glycine as a gating hinge to allow for inner helix bending.
Discussion
• Overall, intra/intersubunit interaction in the open state is not as extensive as closed state due to helix bundle crossing, MO helices, use of detergent, and protein packing.
• In conclusion, KcsA in open state would be similar to that of NaKND19 or MthK.
• NaK (closed) and NaKND19(open) represent the general structure of tetrameric cation channel pore.