Determination of the Equilibrium Constant, Ksp

Determination of the
Equilibrium
Constant, K
sp
, for a
Chemical Reaction
By: Bronson Weston
Background
Information
K sp is a particular type of equilibrium constant called the solubility product constant.
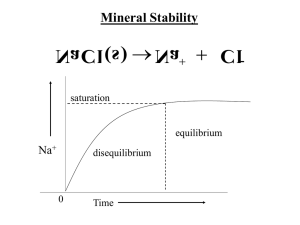
Equilibrium is achieved when an ionic solid dissolves to form a saturated solution. The equilibrium exists between the aqueous ions and the precipitate, an undissolved solid.
A saturated solution contains the maximum concentration of ions of the substance that can dissolve at the solution's temperature.
As the concentration of solute, dissolved ions, increases, so does the rate of reprecipitation. When the rate of reprecipitation equals the rate of dissolution, and there is no more net dissolution of solid, equilibrium is reached.
K
sp
Equation
If given the following reaction:
A n
B m
(s)
n A m+ (aq) + m B n(aq)
The K sp
K sp of the reaction is:
= [A m+ ] n [B n] m
K sp
= molarity of solution
solution is saturated, no precipitate
K sp
< Molarity of Solution
solution is saturated, precipitate is formed
K sp
> Molarity of Solution
solution is unsaturated, no precipitate is formed
Materials
• Calcium nitrate, Ca(NO
3
)
2
, 0.0900
M
• Sodium Hydroxide, NaOH, 0.100 M
• 96- Well Microplate
• Beral pipets labtutorials.org
http://www.odec.ca/projects/2007/meng7l2/G4PicsVideoClips_006.jpg
www.flickr.com
Procedures- Row 1
1. Arrange the Microplate so that you have 12 wells across from left to right
2. Put 5 drops of water in wells #2 through #12 www.gutenberg.org
in the first row
3. Put 5 drops of .0900 M Ca(NO
3
)
2 in well #1 in the first row
4. Add 5 drops of Ca(NO
3
)
2 to well #2
5. Using an empty Beral pipet, mix the solution in Well #2 thoroughly by drawing the solution into the pipet and then squirting it back several times
6. Calculate the molarity of the solution in well #2.
5 drops of 0.0900 M = n moles
5 drops
5 drops Ca(NO
3
)
2
+ 5 drops water = 10 drops n moles = 0.0900 M = 0.0450 M
10 drops 2
7. Using the empty pipet, draw the solution from well #2 and put 5 drops into well #3
8. Put the remaining solution back into well
#2
9. Mix the solution in well #3 with the empty
Beral pipet as before.
10. Continue this serial dilution procedure, adding 5 drops of the previous solution to the 5 drops of water in each well down the row until you close.jpg
fill the last one, well #12.
11. After Mixing the solution in well #12, discard 5 drops
Calculations
12. Determine the concentration of
Ca(NO
3
)
2 solution in each well, using the method used in step 6
Well #1
Well #2
Well #3
Well #4
Well #5
Well #6
Well #7
Well #8
Well #9
Well #10
Well #11
Well #12
0.0900 M
0.0450 M
0.0225 M
0.0113 M
0.00563 M
0.00281 M
0.00141 M
7.03 x 10 -4 M
3.51 x 10 -4 M
1.76 x 10 -4 M
8.79 x 10 -5 M
4.39 x 10 -5 M
More Procedures
13.Place 5 drops of 0.100 M NaOH in each well, #1- #12
14.Use an empty pipet to mix the solution in each well
15.Calculate the concentration of each reactant, Ca +2 and OH , and record the data on a table.
16.Allow three or four minutes for precipitates to form.
17.Observe the pattern of precipitation and record, on the table, which solutions form a precipitate.
I drew this one myself
Well #
Well #1
Well #2
Well #3
Well #4
Well #5
Well #6
Well #7
Well #8
Well #9
Well #10
Well #11
Well #12
Ca +2
0.0450 M
0.0225 M
0.0113 M
0.00563 M
0.00281 M
0.00141 M
7.03 x 10 -4 M
3.51 x 10 -4 M
1.76 x 10 -4 M
8.79 x 10 -5 M
4.39 x 10 -5 M
2.20 x 10 -5 M
OH -
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
0.0500 M
Precipitate
No
No
No
No
No
Yes
Yes
No
No
No
No
No
18.Assume that the first solution, the most concentrated, that does not form a precipitate represents the saturated solution.
19.Calculate the K sp of Ca(OH)
2
, using the concentration of Ca +2 and OH ions in the saturated solution
Calculate the limiting reactant:
Ca +2 + 2OH -
Ca(OH)
2
0.0113 mol Ca +2 x 1.00 mol Ca(OH)
2
1.00 mol Ca +2 )
= 0.0113 mol Ca(OH)
2
0.0500 mol OH x 1.00 mol Ca(OH)
2
2.00 mol OH -
=0.0250 mol Ca(OH)
2
0.0113 mol Ca(OH)
2
< 0.0250 mol Ca(OH)
2
Ca +2 is the limiting reactant
Calculate moles of OH used in the reaction:
0.0113 M Ca +2 x 2.00 M OH -
1.00 M Ca +2
= 0.0226 M OH -
Calculate the K sp
:
K sp
= [A m+ ] n [B n] m
K sp
= [Ca +2 ][OH ] 2
K sp
= [0.0113 M][0.0226 M] 2
Ksp = 5.77 x 10-6
Calculate Percent Error:
[(Experimental Value-Actual Value) / Actual Value] x 100
Actual Value K sp
= 6.5 x 10
Experimental Value K sp
-6
= 5.77 x 10 -6
((5.77 x 10 -6 ) – (6.5 x 10 -6 )) x 100
6.5 x 10 -6
= 11% Error
Explanation
This lab was intended to demonstrate how you can figure out a salt’s solubility constant. When a solution is saturated, it is in equilibrium.
Therefore, when a solution is saturated, we can use the current concentrations of the ions to determine the K sp
. This is why we used the concentrations in the 3 rd well to determine the solubility constant. We were able to assume that the 3 rd well was a saturated solution, because it was the most concentrated solution that did not form a precipitate.
References
http://faculty.kutztown.edu/vitz/limsport/LabMan ual/KSPWeb/KSP.htm
http://www.jesuitnola.org/upload/clark/aplabs.ht
m#Determination%20of%20the%20Solubility%
20Product%20of%20an%20Ionic%20Compoun d
http://mooni.fccj.org/~ethall/2046/ch19/solubilit y.htm
Vonderbrink, Sally Ann. Laboratory
Experiments for Advanced Placement
Chemistry Student Edition. Flinn Scientific, Inc.
Batavia, IL. 1995.