CH13 Notes
advertisement

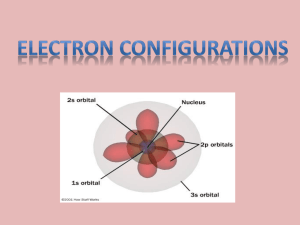
Chapter 13: Electrons in the Atom College Prep Chemistry Orbital Interactive Evolution of Atomic Models 1. John Dalton – indestructible mass - no subatomic particles Evolution of Atomic Models 2. J.J. Thomson – plum-pudding model Discovered electrons electrons stuck into a lump of positively charged material “mint chocolate chip ice cream model” Evolution of Atomic Models 3. Ernest Rutherford nucleus of the atom is positively charged Evidence – gold foil experiment Evolution of Atomic Models 4. Niels Bohr electrons travel in definite orbitals around the nucleus Energy level = the region around the nucleus where the electron is likely to be moving Fixed energy levels analogous to the rungs of a ladder Evolution of Atomic Models Quantum Mechanical Model 5. Describes the probability of finding an electron in a region of space around the nucleus It is impossible to know the exact position and momentum of an electron at the same time Based on probability rather than certainty Instead of traveling in defined orbits as Bohr proposed, electrons actually travel in diffuse clouds around the nucleus Principal Energy Levels (n) Principal (main) energy levels are assigned numbers according to their energy n=1, 2, 3, 4 … Generally, energy increases with increasing n Distance of the electron from the nucleus increases with increasing n Sublevels For each principal energy level, there are one or more sublevels s, p, d, f # of sublevels = the principal energy level (n) For example, 1st principal energy level has 1 sublevel (s) 2nd principal energy level has 2 sublevel (s,p) 3rd principal energy level has 3 sublevels (s,p,d) Sublevels and Orbitals Each sublevel has a specific shape Each sublevel houses a specific # of electron orbitals (“where the electrons live”) Each orbital can contain 2 electrons Sublevels and Orbitals s-sublevel: Spherical in shape One orbital Contains 2e- maximum (2e- per each s orbital) Sublevels and Orbitals p-sublevel: dumbbell in shape 3 orbitals Holds 6 e- maximum (2e- per each p orbital) Sublevels and Orbitals d-sublevel: Double dumbbell in shape 5 orbitals Holds 10 e- maximum (2e- per each d orbital) Sublevels and Orbitals f-sublevel: complex in shape 7 types of f-orbital Holds 14 e- maximum in each orbital (2e- per each f orbital) Atomic Orbital Chart Energy # of Level Sublevels Sublevels # of Orbitals in each Sublevel 1 3 5 n=1 1 s n=2 2 s p n=3 n=4 3 s p d 4 s p d Total # of Electrons 7 2 8 18 f 32 Electron Arrangement in Atoms Electron Configuration: the ways in which electrons are arranged around the nuclei of atoms Gives information about principal energy levels, sublevels, orbitals Three Rules determine electron configurations 1. 2. 3. The Aufbau Principal Hund’s Rule Pauli Exclusion Principle Rules for Electron Configurations Aufbau principle: Electrons enter orbitals of the lowest energy first The s sublevel is always the lowest in energy Rules for Electron Configurations 1. Aufbau principle: Draw your own Aufbau filling diagram 7s 6s 5s 4s 3s 2s 1s 7p 6p 5p 4p 3p 2p 7d 6d 5d 4d 3d 7f 6f 5f 4f (the #4 spelled out has an f) (we see in 3 dimensions!) (two “peas” in a pod) (the #7 spelled out has an s) Rules for Electron Configurations 1. Aufbau principle: Draw your own Aufbau filling diagram 7s 6s 5s 4s 3s 2s 1s 7p 6p 5p 4p 3p 2p 7d 6d 5d 4d 3d 7f 6f 5f 4f Rules for Electron Configurations 2. Hund’s Rule: (“hogs don’t like each other”) Every orbital in a sublevel is singly occupied before any orbital is doubly occupied All of the electrons in singly occupied orbitals have the same spin ↑ ↑ ↑_ Rules for Electron Configurations 3. Pauli Exclusion Principle: an atomic orbital may have a maximum of two electrons Two electrons that occupy the same orbital must have opposite spins designated with Orbital Notation Examples Li B C ↑↓ 1s 2s ↑↓ 1s ↑↓ 2s ↑ ↑↓ 1s ↑↓ 2s ↑ 2p ↑ 2p Hund’s Rule Orbital Notation practice Elements #1-20 Use the Aufbau Diagram Provided Remember all 3 rules! Electron Configurations A shorthand way of identifying the location of electrons C ↑↓ 1s ↑↓ 2s ↑ C 1s2 2s2 2p2 ↑ 2p Electron Configurations Rearranging the e- configurations Scientists rearrange the configurations so that all similar energy levels stay together #21 = Sc (fill with Aufbau first!) 1s22s22p63s23p64s23d1 Then Switch! 1s22s22p63s23p63d14s2 Noble Gas electron configurations Noble gas configurations – look on the periodic table! #11 = Na The noble gas that precedes Na is Ne 1s22s22p6 So instead of 1s22s22p63s1 use [Ne]3s1 Orbital blocks on the Periodic Table Exceptions to electron configurations 24 = Cr # 1s22s22p63s23p64s23d4 ↑↓ ↑ ↑ ↑ ↑ __ Takes less energy to half fill all orbitals 1s22s22p63s23p64s13d5 ↑_ ↑ ↑ ↑ ↑ ↑_ Exceptions to the electron configurations Also true for the rest of column 6 & 11 #29 = Cu Following 1s22s22p63s23p64s23d9 ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑_ Actual the rules configuration 1s22s22p63s23p64s13d10 ↑_ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ Section 13.3 Physics and the Quantum Mechanical Model… Electrons and Light Back to Bohr Bohr’s model is based on atomic emission spectra Atoms only give off light of certain colors (wavelengths) Wave model of light Light is a wave with a frequency, speed and wavelength Emission of light is related to the behavior of electrons in an atom Parts of a Wave (1 of 2) Origin – center line Wavelength (l) – distance from crest to crest Amplitude (A) – distance from origin to crest origin amplitude Parts of a Wave (2 of 2) Frequency SI (f) - # of waves per second (s-1) Unit (Hertz) Hz = 1 s-1 inversely related to wavelength 1 Electromagnetic Radiation (EMR) a form of energy that exhibits wave-like behavior as it travels through space Includes radio waves, microwaves, infrared waves, Visible light, ultraviolet waves, X-rays and gamma rays All waves travel at the speed of light 3.0 X 108 m/s Visible Light A prism separates sunlight into a spectrum of colors Sunlight consists of a continuous range of colors Each color has a specific wavelength and f Red light = lowest f, longest wavelength Electromagnetic Spectrum Wave model of light c=lxf Speed Wavelength Frequency m/s m Hz (hertz) 1/s c = Speed of light = 3.00 x 108 m/s Calculating Frequency Determine the frequency of light with a wavelength of 500 nm? c=lxf l = 500 nm =5 x 10-7 m c = 3.00 x 108 m/s 3.00 x 108 m/s = (5 x 10-7 m) f f = 6.00 x 1014 Hz Calculating Wavelength What is the wavelength of the yellow light emitted by a sodium lamp if the frequency of the radiation is 5.10 x 1014 s-1? Sample Problem Answer Given: f = 5.10 x 1014 s-1 c = 3.00 x 108 m/s Unknown: l Parent Equation: c = f x l Answer: 5.88 x 10-7 m = 588 nm Your turn #1 What is the wavelength of radiation with a frequency of is 1.50 x 1013 s-1? 2.00 x 10 -5 m Active Inspire Your turn #2 What is the frequency of radiation with a wavelength of 5.00 x 10 -6 m? 6.00 x 10 13 s -1 Active Inspire Physics and the Quantum Mechanical Model Max Planck (1858-1947) Discovered a direct relationship between frequency and energy Higher frequency = higher energy E = h x f h = Planck’s Constant = 6.63 x 10-34 J·s Sample Problem What is the energy of a photon with a frequency of 5.00 x 1015 s-1? Sample Problem Answer Given: f = 5.00 x 1015 s-1 h = 6.63 x 10-34 J-s Unknown: E = ? Parent Equation: E = h x f Answer: 3.32 x 10-18 J Your turn #1 What is the energy of radiation with a frequency of is 5.50 x 1014 Hz? Active Inspire Photoelectric Effect Photon - electron in the form of light Ground state – normal position of electron Excited state – electron jumps to higher energy level when energy is applied Photoelectric effect – release of energy in the form of light when electron falls back to ground state Color of light emitted depends on the energy level of the electrons Blue light has a higher energy than red light Photoelectric Effect Atomic Emission Spectrum Elements emit light when electrocuted in gaseous form The light is passed through a prism and an atomic emission spectrum is obtained Atomic emission spectra are NOT continuous like sunlight Each line represents one distinct wavelength and frequency Every element has a unique emission spectra Atomic Emission Spectrum Spectrums When a narrow beam of emitted light is shined through a prism, it is separated into colors of the visible SPECTRUM Types of Spectrums Visible light Spectrum: Continuous range Atomic emission spectrum Light emitted by an element through a prism Every element has a distinct emission spectrum Discontinuous Each line = one frequency Different Elements Have Different Spectrums Video of Spectra via Spectroscopes http://www.mhhe.com/physsci/chemistry/essent ialchemistry/flash/linesp16.swf http://www.flinnsci.com/atomicspectrum More practice problems with energy, frequency, and wavelength Last page of your chapter 13 packet Chapter 13 Review Problems p. 386 #22, 27, 28, 33, 34, 36, 38, 45, 47, 51, 60, 63 Evolution of Atomic Models 5. Quantum Mechanical Model (1 of 2) Quantum of energy = amount of energy required to move an electron from one energy level to the next higher level Energy levels are not equally spaced Levels get closer together the further from the nucleus The further away from the nucleus, the less energy is required for an electron to escape