DRYING - yusronsugiarto
advertisement

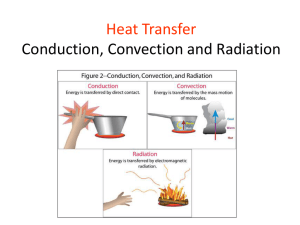
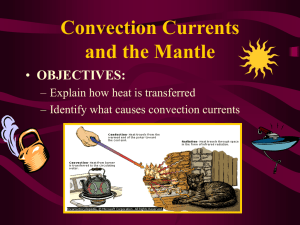
DRYING AND HEAT TRANSFER Yusron Sugiarto, STP, MP, MSc DRYING PROCESSES Drying is perhaps the oldest, most common and most diverse of chemical engineering unit operations. Over four hundred types of dryers have been reported in the literature while over one hundred distinct types are commonly available. Energy consumption in drying ranges from a low value of under five percent for the chemical process industries to thirty five percent for the papermaking operations. DRYING PROCESSES Objective - removal of solvents Contact solids with fluid not saturated with solvent Economics Recover solvent Avoid shipping solvent May avoid spoilage DRYING CURVE DRYING EQUIPMENT CATEGORIES BATCH OR CONTINUOUS DRYING MECHANISM THERMAL http://www.grecobrothers.com/centrifugaldryers/K94.jpg VACUUM FREEZING MECHANICAL http://www.arrowhead-dryers.com/steam-tubedryers.html VACUUM DRYERS SHELF ROTARY http://www.mcgillairpressure.com/index.html CONICAL SHELF DRYERS Batch units Circulate air past trays of fluids Over (cross-circulation) And/or perpendicular to (through-circulation) Can operate under vacuum Long batch cycles (4 - 48 hours) are common Primary uses Plastics Metals Chemicals Pharmaceuticals Foods http://www.bocedwards.com/ind ex.cfm?ProcessVacuum/pharmac eutical-upgrades.cfm~content TUNNEL DRYERS Move material on belt or conveyor through drying zone Used for a wide range of free-flowing particulates (granular, flake or fibrous) Used for pastes and filter cakes with even application to belt Drying times approximately 5 - 120 minutes Large capacity is practical with these units http://www.dryer.com/Columbia%20Flyer%2005%20.pdf ROTARY DRYERS Drop solids through counter current flowing hot gases Can be lined with refractory to allow very high temperature operation High volume with wide stable operating range Residence times typically measured in minutes http://www.siko.co.id/images/kiln.jpg DRUM AND WIPED-FILM DRYERS Drum dryers Thin film dryers with indirect heating Slurry applied to drum and dried solid removed (see fig. 9.2-4) Wiped film dryers Inverse of drum dryer with internal wiper to apply film to vertical surface Material leaving dryer must be free flowing High thermal efficiency FEED WIPED FILM DRYER FINISH DRYER http://www.atlascoffee.com/imgz/1br/1br07.jpg FREEZE DRYERS USED FOR BIOLOGICALS USE SUBLIMATION http://www.niroinc.com/html/ch emical/freezedryers.html http://www.pharmaceuticaltechnology.com/contractors/process_automa tion/telstar/telstar1.html FLASH OR SPRAY DRYERS Contact flow with concurrent flow of hot air Solids may be entrained Solids may fall through air May incorporate cyclone May incorporate sprayer to produce slurry droplets May be included on tall tower (prilling) operation http://www.ocsd.co.jp/english/exampleusage/ index01.html FLUID BED DRYERS Suspend solids in hot air stream Gentle processing – no degradation Uniformity of process conditions Fed slurry from a centrifuge Recover fines with either cylcone, filter or esp and re-slurry http://www.niroinc.com /html/drying/fluidbed.h tml FLUID BED DRYER EXAMPLE http://www.barr-rosin.com/english/products/fluid-bed-dryer.htm Heat Transfer Heat always moves from a warmer place to a cooler place. Hot objects in a cooler room will cool to room temperature. Cold objects in a warmer room will heat up to room temperature. Question If a cup of coffee and a red popsickle were left on the table in this room what would happen to them? Why? The cup of coffee will cool until it reaches room temperature. The popsickle will melt and then the liquid will warm to room temperature. Basics to heat transfer (1) Heat (Q) = a form of energy [ J or Btu ] (2) Rate of heat transfer (q) = amount of heat (J, Btu) unit time (s ,hr) *** (J/s = Watts) (3) Nature of heat flow “Net heat flow is always in the direction of temperature decrease” (4) Heat flux = rate of heat flow per unit area = q/A = Q t X [J/s m2] A (5) Temperature gradient = changes of temperature with distance, i.e. for x direction = dT/dx [°C/m] Heat Transfer Methods Heat transfers in three ways: Conduction Convection Radiation Conduction When you heat a metal strip at one end, the heat travels to the other end. As you heat the metal, the particles vibrate, these vibrations make the adjacent particles vibrate, and so on and so on, the vibrations are passed along the metal and so is the heat. We call this? Conduction Metals are different The outer e______ lectrons of metal atoms drift, and are free to move. When the metal is heated, this ‘sea of electrons’ gain k_____ energy and transfer it inetic throughout the metal. Insulators, such as w___ and ood p____, do not lastic have this ‘sea of electrons’ which is why they do not conduct heat as well as metals. Why does metal feel colder than wood, if they are both at the same temperature? Metal is a conductor, wood is an insulator. Metal conducts the heat away from your hands. Wood does not conduct the heat away from your hands as well as the metal, so the wood feels warmer than the metal. * Heat Transfer by Conduction - If temperature gradient exists in a continuous substance (solid, fluid and gas), heat can flow without observable motion of matter. - Heat flux is oppositely proportional to the temperature gradient (Fourier’s law) dq dT k dA dx where, q = A = T = k = ……………… ( I ) rate of heat flow in direction normal to surface surface area temperature x = distance normal to surface proportionality constant or thermal conductivity Convection What happens to the particles in a liquid or a gas when you heat them? The particles spread out and become less dense. What A liquid is afluid fluid? or gas.movement. This effects Fluid movement Cooler, more d____, ense fluids armer sink through w_____, less dense fluids. In effect, warmer liquids and gases r___ up. ise Cooler liquids and gases s___. ink Water movement Cools at the surface Cooler water sinks Convection current Hot water rises Why is it windy at the seaside? Cold air sinks Where is the freezer compartment put in a fridge? It is put at the top, because cool air sinks, so it cools the food on the way down. Freezer compartment It is warmer at the bottom, so this warmer air rises and a convection current is set up. * Heat Transfer by Convection - Flow of heat associated with the movement of fluid wall Cold fluid q High Temperature - Convective flux T (Newton’s law of cooling) q h (Ts Tf ) A ……………….. ( II ) where, Ts = surface temperature Tf = bulk temperature of fluid, far from surface h = heat-transfer coefficient * Heat Transfer by Convection (next) **unlike k, h depends not only on thermal properties of fluid but also flow patterns** ้ อ convection แบ่งออกเป็ น 2 แบบ ดังนี คื - Force convection - Natural convection Temperature gradient in fluid buoyancy forces flow The third method of heat transfer How does heat energy get from the Sun to the Earth? ? There are no particles between the Sun and the Earth so it CANNOT travel by conduction or by convection. RADIATION Radiation Radiation travels in straight lines True/False Radiation can travel through a vacuum True/False Radiation requires particles to travel True/False Radiation travels at the speed of light True/False Absorption experiment Four containers were placed equidistant from a heater. Which container would have the warmest water after ten minutes? Dull metal Shiny metal Shiny black Dull black dull black container would be the warmest after ten minutes The __________ shiny metal because its surface absorbs heat radiation _______ the best. The _________ container would be the coolest because it is the poorest at absorbing heat radiation. __________ *Heat Transfer by Radiation Transfer of energy through space by electromagnetic waves Through empty space, energy not transformed nor diverted Through matters, transmitted, reflected, or absorbed Absorbed energy is in form of heat Energy emitted by a black body (absolute temp.)4 W εσT b 4 Wb T 4 ……….. (III) where, Wb = rate of radiant energy emission per unit /area = Stefan-Boltzmann constant T = absolute temperature = emissivity