Gram-staining - BMC Dentists 2011
advertisement

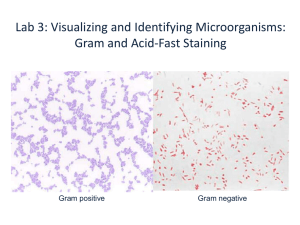
Gram staining Gram stain kits is used to stain microorganisms from cultures and specimens by the differential gram method. The Gram stain is used to differentiate bacteria into two groups based on the cell color after staining: 1. Gram +ve bacteria which are bluish purple. 2. Gram ₋ve bacteria which are pinkish red. Requirements for Gram staining: 1. Glass slides. 2. Bunsen flame. 3. Gram stain kit. 4. Microscope. 5. Wire loop. Reagents in Gram stain kit: 1. Gram’s crystal violet solution. 2. Gram’s iodine solution. 3. Gram’s decolorizing solution. 4. Gram’s safranine solution. Procedure for making a bacterial smear Gram Staining method: Flood the fixed smear with Gram's crystal violet Solution. Let stand for 2 minutes. Pour off the stain and gently wash with tape water from a faucet or a plastic water bottle. Flood with Gram's iodine Solution. Allow it to remain for 2 minutes (function as mordant which increases interaction between the cell and dye so that cell is stained more strongly. Pour off the iodine solution and gently wash with tape water. Shake off the excess water from the surface. Decolorize with Gram's Decolorizer Solution (95% Ethanol) for 3 seconds until the blue dye no longer flows from the smear. Further delay will cause excess decolorization in the gram-positive cells. Gently wash the smear with tape water. Counterstain with Gram's safranin solution for 2 minutes. Wash off the safranin solution with water. The slide is to be shaken to remove most of the water and air-dried. Examine the finished slide under a microscope . Gram +ve Results of Gram’ reaction Gram -ve Ziehl Neelsen staining The Ziehl-Neelsen (Zn) technique is used to stain Mycobacterium species including M. tuberculosis, and M. leprae. Mycobacteria, unlike most other bacteria, do not stain well by the Gram technique. The stain binds to the mycolic acid in the mycobacterial cell wall. After staining, an acid decolorizing solution is applied. This removes the red dye from the background cells, tissue fibers, and any organisms in the smear except mycobacteria which retain (hold fast to) the dye and are therefore referred to as acid fast bacilli “AFB”. Hot and cold Zn techniques In the ‘hot’ Zn technique the phenolic-carbol fuchsin stain is heated to enable the dye to penetrate the waxy mycobacterial cell wall. This heating releases phenol vapours which are TOXIC. Techniques which do not heat the stain are referred to as ‘cold’ techniques. Requirements Glass slide Bacterial specimen Hot Plate Methanol for smear fixation Staining reagents i.e. a) Primary stain Carbol fuchsin (Red color) b) Decolorizer (95% Ethanol + 3% HCl) and c) Counter stain Methylene blue Microscope 1. Steps in Staining: Smear preparation and Fixation Prepare thin smear of given sample on a glass slide. Allow the slide to air dry. Fix with methanol: Cover the slide with methanol for 10-15 minutes until the alcohol completely evaporates. In this way Mycobacterium becomes completely inactive and attached firmly to the slide. 2. Staining (i) Application of Carbol Fuchsin: Two methods are used i.e. a) Hot Method Cover the smear with Carbol Fuchsin and heat the slide by placing it on Hot plate for 3-5 minutes. Do not allow the stain to boil and dry but it should only evaporate. By this way stain easily goes into the cell. b) Cold Method The smear is flooded with Carbol Fuchsin along with a wetting agent (Turgitol). Turgitol reduces surface tension between the cell wall of mycobacteria and the stain. So that stain easily goes into the cell. Following application of primary stain, all cells will appear red. (ii) Rinse with tap water. (iii) Add the acid-alcohol decolorizing (95% ethanol and 3% HCl) slowly drop wise until the dye no longer runs off from the smear (20-30 seconds). (iv) Wash well with tap water. (v) Counterstain with methylene blue for 2 minute. (vi) Wash with water, air dry and observe using oil immersion (at 100X) lens. Kinyoun- Gabett staining kit Requirements 1. Kinyoun- Gabett staining kit. 2. Glass slides 3. Thermal block (for the hot method). 4. Methanol. 5. Microscope. Kinyoun- Gabett staining Kit: 1) Kinyoun solution: 2) Gabbett solution: 1. Methylene blue. 1. Basic fuchsin. 2. Sulfuric acid. 2. Phenol. 3. Ethyl alcohol. 3. Ethyl alcohol. 4. Demineralized 4. Demineralized water. water. Kinyoun- Gabett staining method Smear preparation and fixation: 1. Smear the sample to be examined in a thin layer on a clean slide. 2. Allow to dry. 3. Fix with methanol: cover the slide with alcohol for 10- 15 minutes until the alcohol completely evaporates. A. Cold method 1. Place the slide in a staining dish filled with Kinyoun solution for 3 hours. 2. Rinse with water. 3. Cover the smear with Gabbett solution for 5 minutes. 4. Rinse with water. 7. Wipe the back of the slide clean, and place it in a draining rack for the smear to air-dry. B. Hot method 1. Place the slide on a thermal block. 2. Cover the smear with filter paper which is slightly smaller than the slide. 3. Float the paper with Kinyoun solution. 4. Heat until steam is given off. 5. Leave in contact for 10 minutes. Regularly flood the filter paper to prevent drying. 6. Rinse with water. 7. Cover with Gabbett solution. 8. Rinse with water. 9. Air dry. Results AFB . . . . . . . . . . . . . . . . . . . . Red, straight or slightly curved rods, occurring singly or in small groups Reporting of sputum smears 1. More than 10 AFB/field . . . . . . . . . . . report +++ 2. 1–10 AFB/field . . . . . . . . Report ++ 3. 10–100 AFB/100 fields . . report + 4. 1–9 AFB/100 fields . . . . . report the exact number 5. When no AFB are seen after examining 100 fields: Report the smear as ‘No AFB seen’. Do not report Negative’ because organisms may be present but not seen. Up to three specimens (one collected as an early morning specimen) may need to be examined to detect M. tuberculosis in sputum.