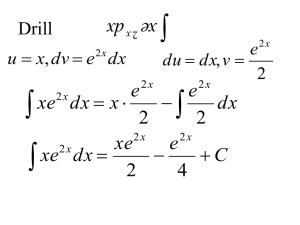Unit 6 - Earth History
advertisement

Chapter 6 review 1. UNIFORMITARIANISM: -The processes that shape the Earth today are the same processes that occurred in the geologic past “The present is the key to the past” 2. ORIGINAL HORIZONTALITY -Sedimentary rock layers are ALWAYS laid down in horizontal layers, until some other process alters them 3. SUPERPOSITION -The bottom layer of horizontal sedimentary rock layers is always the oldest, UNLESS the layers have been overturned or have had older rock formed on top of it *The lower layers must first be in place before the next layer can be deposited YOUNGEST OLDEST Extrusion: When molten rock flows on the Earth’s surface forming an igneous rock Intrusion: Occurs when magma squeezes between layers of pre-existing rock beneath the Earth’s surface -Causes surrounding layers to metamorphose (Contact metamorphism) FOLDS AND FAULTS Fold: Folds and Faults are always younger than the original rock layers -bends in rock layers produced by movements of the earth’s crust Faults: -breaks in the rock where shifting of rock layers has occurred, often associated with earthquakes -Preserved remains or trace evidence of a plant or animal living in the past Fossils reveal clues to ancient environments Mrs. Sharp Index Fossils: Fossils that are found geographically widespread and lived for a short period of time By comparing index fossils in various locations on Earth, it is possible to correlate (match) the relative ages of the rocks in which they appear “Geographically widespread” means the index fossils are found in more than one rock column Lived for a “short period of time” means the index fossils are found in only one layer What is Correlation? When geologists try to match rock outcrops in different locations to see if they formed at the same time. How to correlate (match) rock layers: Similarity of rock types Matching index fossils Volcanic ash layers used as time markers Relative Age of rocks: (sequence) The age of a rock layer in comparison to its surrounding layer -Uses rock similarity, fossil evidence, and volcanic time markers to determine its order of occurrence Absolute Age of rocks: (true age) The age of a rock layer in years -Uses radiometric dating to determine age--radioactive decay Radioactive decay is the process by which the natural breakdown of unstable atoms of an element occurs, releasing particles and energy (heat), and changes that element’s atoms into a new element Example: it takes 4.5 billion years for uranium 238 to change into lead 206 Radioactive decay is NOT AFFECTED by temperature (heat) or pressure! Radioactive decay occurs at a constant rate known as half-life. HALF-LIFE: Half-life is the rate (time) it takes for one-half of the amount of original material to decay If we know the half-life of a radioactive material, the age of the material can be determined by measuring the amount of decayed material in the sample. ESRT pg. 1 Radioactive Decay Data chart Some radioactive substances have a Short half-life: Carbon 14 -Good for dating recent organic remains (between 1,000-50,0000 yrs.) 238 206 Uranium decays to Lead 238 Long half-life: Uranium -Good for dating much older rocks (a very long half-life) - it takes 4.6 billion years for uranium to decay to lead Example: The amount of Carbon-14 remaining in a fossil is 0.5 grams. How old is the fossil? An equal sample of an existing organism shows the original amount of Carbon-14 was 2.0 grams. (2.0g _____ _____) 1.How many half-lives did the sample undergo? 2.Multiply this by the half-life for Carbon-14 (ESRT) Example: The amount of Carbon-14 remaining in a fossil is 0.5 grams. How old is the fossil? An equal sample of an existing organism shows the original amount of Carbon-14 was 2.0 grams. (2.0g 1.0g 0.5g) 1.How many half-lives did the sample undergo? 2.Multiply this by the half-life for Carbon-14 (ESRT) Answer: 2 x 5,700 = 11,400 yrs. Example: The amount of Carbon-14 remaining in a fossil is 0.5 grams. How old is the fossil? An equal sample of an existing organism shows the original amount of Carbon-14 was 2.0 grams. (2.0g 1.0g 0.5g) 1.How many half-lives did the sample undergo? 2 2.Multiply this by the half-life for Carbon-14 (ESRT) Example: The amount of Carbon-14 remaining in a fossil is 0.5 grams. How old is the fossil? An equal sample of an existing organism shows the original amount of Carbon-14 was 2.0 grams. (2.0g 1.0g 0.5g) 1.How many half-lives did the sample undergo? 2 2.Multiply this by the half-life for Carbon-14 (ESRT) Example: The amount of Carbon-14 remaining in a fossil is 0.5 grams. How old is the fossil? An equal sample of an existing organism shows the original amount of Carbon-14 was 2.0 grams. (2.0g 1.0g 0.5g) 1.How many half-lives did the sample undergo? (how many times did it decay) 2 half-lives 2.Multiply this number by the half-life for Carbon-14 (ESRT) Answer: 2 x 5,700 yrs. = 11,400 yrs.