Chapter 3 - Evangel University
advertisement

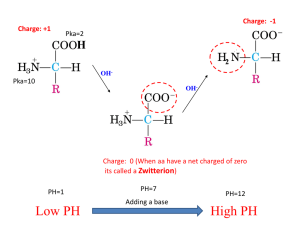
Mary K. Campbell Shawn O. Farrell http://academic.cengage.com/chemistry/campbell Chapter Three Amino Acids and Peptides Paul D. Adams University of Arkansas Amino Acids • Amino acid: a compound that contains both an ____________ ____________ and a ____________ ____________ • -Amino acid has an amino group attached to the carbon adjacent to the carboxyl group • -carbon also bound to side chain group, R • R gives identity to amino acid • Two steroisomers of amino acids are designated L- or D-. Based on similarity to glyceraldehdye (Figure 3.2) Amino Acid Structure and Properties • With the exception of ____________, all proteinderived amino acids have at least one ____________ (the -carbon) and are ____________ (stereoisomers) • the vast majority of -amino acids have the ________ -configuration at the -carbon (Proline is usually ___) • Side-chain carbons in other amino acids designated with Greek symbols, starting at a carbon (…etc) • Amino acids can be referred to by three-letter or oneletter codes. Table 3.1 (KNOW THESE) Individual Amino Acids • Group A: __________ side chains- Ala, Val, Leu, Ile, Pro. Phe, Trp, Met. • Ala, Val, Leu, Ile, Pro- contain aliphatic hydrocarbon group. Pro has cyclic structure. • Phe- hydrocarbon aromatic ring. • Trp- Indole ring side chain, aromatic. • Met- Sulfur atom in side chain. Amino Acids (cont’d) • Group B: _______ _______ side chains- Ser, Thr, Tyr, Cys, Glu, Asn • Ser, Thr- Side chain is polar hydroxyl group • Tyr- hydroxyl group bonded to aromatic hydrocarbon group • Cys- Side chain contains thiol group (-SH) • Gln, Asn- contain amide bonds in side chain Amino Acids (cont’d) • • • • Group C: ____________ Side Chains: Glu, Asp Both have a carboxyl group in side chain Can lose a proton, forming a carboxylate ion These amino acids are negatively charged at neutral pH Amino Acids (cont’d) • • • • • Group D: ___________ side chains: His, Lys, Arg Side chains are positively charged at pH 7 Arg-side chain is a guanidino group His-side chain is an imidazole group Lys-side chain NH3 group is attached to an aliphatic hydrocarbon chain Amino acid summary Important structural features: 1. All 20 are -amino acids 2. For 19 of the 20, the -amino group is ________; for proline, it is __________ 3. With the exception of ___________, the -carbon of each is a stereocenter 4. ____________ and ____________ contain a second stereocenter 5. 3- and 1-letter codes in Table 3.1. Uncommon Amino Acids • Each derived from a common amino acid by a modification • ___________ and ___________ are found in only a few connective tissues such as collagen • ________ is found only in the thyroid gland Ionization of Amino Acids • In amino acids, carboxyl group (-) and amino group (+) are ________________ at neutral pH. • In free amino acids -carboxyl, and a-amino groups have ________________ protons. Some side chains do as well Ionization of Amino Acids • Remember, amino acids without charged groups on side chain exist in neutral solution as _____________ with no net charge Titration of Amino Acids • When an amino acid is titrated, the titration curve represents the reaction of each functional group with the hydroxide ion Titration of alanine with NaOH Titration of histidine with NaOH Acidity: -COOH Groups • The average pKa of an -carboxyl group is 2.19, which makes them considerably stronger acids than acetic acid (pKa 4.76) • the greater acidity of the amino acid carboxyl group is due to the _________ ____________ ____________ of the -NH3+ group Basicity: -NH3+ groups • The average value of pKa for an -NH3+ group is 9.47, compared with a value of 10.76 for a 2° alkylammonium ion Basicity (cont’d) Guanidine Group • The side chain of arginine is a considerably stronger base than an ________ amine • basicity of the guanido group is attributed to the large ________ ________ of the protonated form relative to the neutral form Imidazole Group • The side chain imidazole group of ________ is a ____________ ____________ ____________ Ionization vs pH • Given the value of pKa of each functional group, we can calculate the ratio of each acid to its conjugate base as a function of pH • Consider the ionization of an -COOH C O O H + pK a = 2.00 H2 O C O O - + H3 O + • writing the acid ionization constant and rearranging terms gives (remember Ch. 2) Ka = [ H 3 O + ] [ -COO [ -COO H] - [ -COO ] or - ] [ -COO H] = Ka + [ H 3O ] Ionization vs pH (cont’d) • substituting the value of Ka (1 x 10-2) for the _______ ______ concentration at pH 7.0 (1.0 x 10-7) gives [ -COO - ] [ -COO H] = Ka + [ H 3O ] 1.00 x 10 -2 1.00 x 10 -7 = = 1.00 x 10 5 • at pH 7.0, the -carboxyl group is virtually ___% in the ionized or conjugate base form, and has a net charge of _______________ • we can repeat this calculation at any pH and determine the ratio of [-COO-] to [-COOH] and the net charge on the -carboxyl at that pH Ionization vs pH (cont’d) • We can also calculate the ratio of acid to conjugate base for an -NH3+ group; for this calculation, assume a value 10.0 for pKa N H 3 + + H2 O pK a = 10.00 N H 2 + H3 O+ • writing the acid ionization constant and rearranging gives [ -NH [ -NH 2 ] + 3 Ka = ] [H 3 O+ ] Ionization vs pH • substituting values for Ka of an -NH3+ group and the hydrogen ion concentration at pH 7.0 gives [ -NH [ -NH 2] + 3 = ] Ka [H + O ] 3 = 1.00 x 10 -10 1.00 x 10 -7 = 1.00 x 10 -3 • at pH 7.0, the ratio of -NH2 to -NH3 + is approximately 1 to 1000 • at this pH, an -amino group is 99.9% in the ______ or _______ form and has a charge of ___ Henderson-Hasselbalch Equation • We have calculated the ratio of _______________ to ____________ for an ________________ group and an ____________ group at pH 7.0 • We can do this for any weak acid and its conjugate base at any pH using the Henderson-Hasselbalch equation (Ch. 2) pH = pK a + log [conjugate base] [weak acid] Isoelectric pH • Isoelectric pH, pI: the pH at which the majority of molecules of a compound in solution have ___________________ • the pI for glycine, for example, falls midway between the pKa values for the carboxyl and amino groups pI = = 1 2 ( p K a CO O H + p K a N H 3 + ) 1 (2.35 + 9.78) = 6.06 2 • Isoelectric pH values for the 20 protein-derived amino acids are given in Table 3.2 Electrophoresis • Electrophoresis: the process of separating compounds on the basis of their ____________ • electrophoresis of amino acids can be carried out using paper, starch, agar, certain plastics, and cellulose acetate as solid supports • in paper electrophoresis, a paper strip saturated with an aqueous buffer of predetermined pH serves as a bridge between two electrode vessels Peptide Bonds • Individual amino acids can be linked by forming covalent bonds. • Peptide bond: the special name given to the ____________ bond between the _________ group of one amino acid and the ____________ group of another amino acid Geometry of Peptide Bond • the four atoms of a peptide bond and the two alpha carbons joined to it lie in a plane with bond angles of 120° about C and N • to account for this geometry, a peptide bond is most accurately represented as a hybrid of two contributing structures (____________ structures) • the hybrid has considerable C-N ___________ bond character and rotation about the peptide bond is ____________ • See Figure 3.10 Peptides • ____________ : the name given to a short polymer of amino acids joined by peptide bonds; they are classified by the number of amino acids in the chain • ____________ : a molecule containing two amino acids joined by a peptide bond • ____________ : a molecule containing three amino acids joined by peptide bonds • ____________ : a macromolecule containing many amino acids joined by peptide bonds • ____________ : a biological macromolecule of molecular weight 5000 g/mol or greater, consisting of one or more polypeptide chains Peptides with Physiological Activity Peptides with Physiological Activity (cont’d)