Interfaces_1b
advertisement

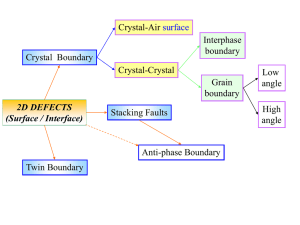
Crystal-Air surface Interphase boundary Crystal Boundary Crystal-Crystal Grain boundary 2D DEFECTS (Surface / Interface) Stacking Faults Anti-phase Boundary Twin Boundary Low angle High angle Low angle High angle Based on angle of rotation Homophase Based on axis Based on Lattice Models Twist Tilt Mixed Special Based on Geometry of the Boundary plane Random Curved Faceted Mixed CSL/Other Curtesy S. Van Tenderloo Picture ARM-UC Berkeley4 Low angle Semicoherent High angle Incoherent Based on angle of rotation Interphase Based on axis Based on Lattice Models Twist Tilt Mixed Special Based on Geometry of the Boundary plane Epitaxial/Coherent Random Curved Faceted Mixed Wulff-type constructions Coherence at interfaces • Coherent interface means an interface in which the atoms match up on a 1-to-1 basis (even if some elastic strain is present). • Incoherent interface means an interface in which the atomic structure is disordered. • Semi-coherent interface means an interface in which the atoms match up, but only on a local basis, with defects (dislocations) in between. Coherent interfaces • Coherent interface means an interface in which the atoms match up on a 1-to-1 basis (even if some elastic strain is present). • Near identical lattice parameters, often thin layers of A on B Incoherent interfaces • Incoherent interface means an interface in which the atomic structure is disordered. • General case, analogous to a general high-angle grain boundary (roughly) Semi-coherent interfaces • Semi-coherent interface means an interface in which the atoms match up, but only on a local basis, with defects (dislocations) in between. • Comparable to a low-angle grain boundary with a dislocation array (now called misfit dislocations) Epitaxy Britannica Concise Encyclopedia: epitaxy Process of growing a crystal of a particular orientation on top of another crystal. If both crystals are of the same material, the process is known as homoepitaxy; if the materials are different, it is known as heteroepitaxy. Common types of epitaxy include vapour phase, liquid phase, and solid phase, according to the source of the atoms being arranged on the substrate. Comment 1: “growth” is not needed here… Comment 2: often used more generally than this Main Types of Epitaxy • Homoepitaxy – Growth of material on the same substrate (Si on Si) • Pseudomorphic growth – Material adopts the lattice of substrate/matrix • Coincidence – Material has certain spacings common with substrate/matrix – Similar to CSL • Cube-Cube – Major orientations are parallel, e.g. [001]A//[001] substrate Heteroepitaxial growth modes Frank-van der Merwe Volmer-Weber Stranski-Krastanov trade surface for interface relieve stress 2 1 layer-by-layer Pseudomorphic Growth Pseudomorphic Growth • Consider a layer of “A” on “B”, of thickness t • Take z normal to film, x in plane • Suppose that lattice of A is larger than that of B, and would match that of B is strained by exx along x • Strain energy scales as texx2 (I leave to you to work this out in detail…) per unit area Interface Energy • If A matches the lattice of B, the “bonding” will be good • Energy of interface per unit area is gAB • Total energy of system – E = t*exx2 + gAB Alternative • Hetero epitaxial growth (“lattice-mismatched” growth) permits the fabrication of dissimilar materials on the same substrate • Strain in the growing film depends on thickness and mismatch Thin layer - the film will elastically deform to match the in-plane lattice parameter of the substrate Thick layer - film will revert to its unstrained lattice parameter, with misfit dislocations at the interface with the substrate Alternative, dislocations • Put dislocations at the interfaces of Burgers vector b, separation L • Assume that these remove all the strain – b/L = exx • Energy of dislocations per unit area will scale as b2/L (better, use Read-Shockley model or similar, Frank-Van Der Merwe) – Note: no t dependence T T T T Energy Balance • For “phase transition” pseudomorphic to dislocations DE = -C1*t*exx2 + C2(b2/L) 2.5 Strained Relaxed 2 1.5 1 0.5 0 0 0.5 1 1.5 2 Dislocation Standoff Dislocation energy scales with shear modulus T m1 T T m2 T T T T T T m1 > m2 T T m1 = m2 m1 < m2 T Energy Balance • Better, consider a half dislocation loop growing in (kinetics) • Energy of loop = pRC2b2 • Strain energy relieved = C1pR2/2exx2 • For transition (remove p & 1/2) DE = -C1R2exx2 + RC2b2 4.5 Strained 4 Relaxed 3.5 3 R 2.5 2 1.5 1 0.5 0 0 0.5 1 1.5 2 Classic Nucleation Problem 0.5 0 0 -0.5 -1 -1.5 -2 -2.5 0.5 1 1.5 2 Islands Similar Cases • Thin films/precipitates can have different structures – Energy for phase change < interface energy DE = C1*V + C2V2/3(gA-gB) B1-AlN VN 3 nm Epitaxial Stabilization of B1-AlN in AlN/VN Al Superlattices N a VN w-AlN zb-AlN B1-AlN 5 a relative to wurtzite AlN Energy of B1-AlN and zb-AlN vs. underlayer lattice constant (not including the interfacial energy). [Madan et al.] Total energy per unitcell (eV) TiN 4 B1 3 2 1 zinc-blende 0 4.0 4.2 4.4 4.6 4.8 Underlayer Lattice Constant (Å) Similar Cases • Nanoparticles can have different structures – Energy for elastic strain < surface energy DE = C1*V + (CA-CB) V2/3gA Stranski-Krastanow Growth • Formation of 3D structures (q-dots) preceded by wetting layer • Relieve strain energy, increase surface energy DE = C1V2/3+C2V Comments • Similar to CSL boundaries, one can have dislocations of the coherency between the two materials at an interface • A step at the interface is normally a different type of dislocation – sessile (immobile) • There is more…. Low angle Semicoherent High angle Incoherent Based on angle of rotation Interphase Based on axis Based on Lattice Models Twist Tilt Mixed Special Based on Geometry of the Boundary plane Epitaxial/Coherent Random Curved Faceted Mixed Wulff-type constructions Curtesy S. Van Tenderloo Picture ARM-UC Berkeley30