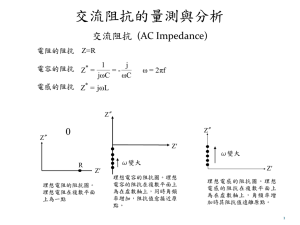
投影片 1
advertisement
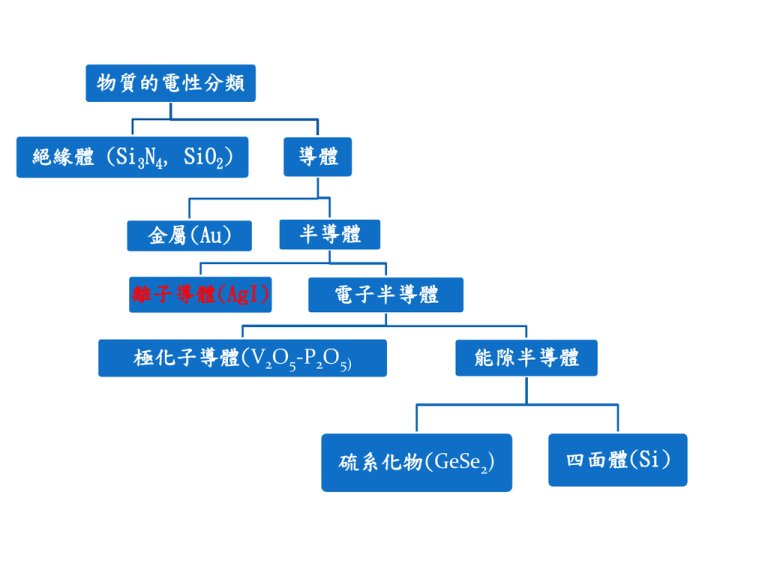
物質的電性分類 絕緣體 (Si3N4, SiO2) 金屬(Au) 離子導體(AgI) 導體 半導體 電子半導體 極化子導體(V2O5-P2O5) 能隙半導體 硫系化物(GeSe2) 四面體(Si) 電子的導電率 ne 2τ 1 1 1 σ= , = + m τ τ L τi τL: collision time for scattering by phonons, 1 1 σ ∝ for high temp (T > θ D ), σ ∝ for low temp (T << θ D ), 5 T T θ D is Debye temp τi: collision time for scattering by imperfections. σ independent of temp σ T -5 T T -1 半導體的導電率 -Eg / 2kT -Eg / 2kT 3 σ = nμq = aoT e = σoe 離子的導電率 1 -Eσ / kT -E / kT σ = nμq = a o e = σoe σ T σo 為常數,此關係稱為Arrhenius Equation,其中k為波茲曼常數 (Boltzmann’s Constant),Eσ為由導電度產生的活化能,T為絕對溫度。 斜率=log(e)E σ/k logσ 1/T For any material and charge carrier, the conductivity is given by σ=Σnieii where ni is the number of charge carriers of species i, ei is their charge and i their mobility. Migration of ions does not occur to any appreciable extent in most ionic and covalent solids such as oxides and halides. Rather, the atoms tend to be essentially fixed on their lattice sites and can only move via crystal defects. Only at high temperatures, where the defect concentrations become quite large and the atoms have a lot of thermal energy, does the conductivity become appreciable, e. g. the conductivity of NaCl at ~800oC, just below its melting point, is ~10-3 (Ωcm)-1, whereas at room temperature, pure NaCl is an insulator. In contrast, there exists a group of solids called, solid electrolytes, fast ion conductors and superionic conductors, in which one of the sets of ions can move quite easily. Such materials often have rather special crystal structures in that there are open tunnels or layers through which the mobile ions may move. The conductivity values, e. g. ~10-3 (Ωcm)-1, for Na+ ion migration in β-alumina at 25 oC, are comparable to those observed for strong liquid electrolytes. 極化子傳導 許多含過渡元素的氧化物玻璃,其過渡元素在玻璃中有兩種價態。例如鐵離子在氧化 物玻璃中以Fe+3與Fe+4同時存在,而釩離子也經常以V+4與V+5同時存在。電子可以在V+4與V+5 之間躍遷(hopping),此躍遷現象會造成導電的效果,但是此類過渡元素間不同價電子的躍 遷往往會引起晶格變形的移動。在此同時這種晶格變形與電子共同移動的現象,可以看成是 一種粒子稱為極化子(polaron),而實際上極化子是電子與聲子之間的交互作用。 極化子產生機制。 中間為一電子,電 子可極化周圍環境 使附近原子重新排 列而產生極化子 (a) θD σ = σo exp[ -Eσ / kT ] for T > 2 θD 1 / 4 σ = σo exp[ -(To / T) ] for T < 2 (b) Mn+ M(n+1)+ (c) M(n+1)+ M(n+1)+ 小極化子的移動過程示意圖 M(n+1)+ Mn+ Typical ionic crystals NaCl + Migration of Na vacancies in NaCl The magnitude of the ionic conductivity of NaCl depends on the number of cation vacancies present, Vacancies are normally created by one of two methods. On heating a crystal, the number of vacancies present in thermodynamic equilibrium increases exponentially and this is the number that is intrinsic to the pure crystals. Vacancies may also be created by adding impurities, e. g. addition of small amount of MnCl2, this will yield solid solution with fomula,Na1-2xMnxCl. Schematic ionic conductivity of doped NaCl crystal. + Pathway for Na migration in NaCl The radius of Na+ is rNa+~0.95A . The jumping of ion Na+ in NaCl is a difficult and complicate process. First , the ion has to squeeze through a narrow triangular interstice of radius r’ (=0.45A) , whereupon it finds itself in a small tetrahedral interstitial site of radius 0.59A. The residence time here is quite short since this site has a hostile environment with two Na+ ions, 1 and 2, at a distance of 2.44A as well as four Cl- ions at the same distance. The ion leaves by squeezing through another gap of radius 0.45A to occupy the vacant octahedral site on the other side. Ionic conductivity of “pure “ NaCl single crystal as function of temperature Region I intrinsic conductivity II extrinsic conductivity I’ anion vacancy or Debye-Huckel interaction III formation of defect complex cation/anion vacancy pairs or cation vacancy/aliovalent cation impurity pairs Schottky pair defect Conductivity in NaCl crystals Process Activation energy (eV) Migration of Na+, Em Migration of ClFormation of Schottky pair Dissociation of vacancy Dissociation of cation vacancy -Mn+2 pair 0.65~0.85 0.90~1.10 2.18~2.38 ~1.3 0.27~0.50 Two Frenkel defects Typical ionic crystals AgCl The predominant defects in AgCl are Frenkel detects. Formation energy of Frenkel detects is 1.24eV, migration energy of cation vacancy is 0.27~0.34eV, migration energy of interstitial Ag+ is 0.05~0.16eV. It has been found experimentally that mechanism 2 is the operative mechanism. intrinsic extrinsic The effect of aliovalent cation impurities on the extrinsic conductivity region of AgCl is different to that observed in NaCl. The presence of Cd2+ again increases the number of cation vacancies, but because the product of the concentrations of cation vacancies and Ag+ interstitials is constant, the interstitial Ag+ concentration must decrease with increasing Cd+2 concentration. Addition of Cd2+ therefore leads to a reduction in the concentration of the more mobile species. An extrinsic region at lower temperatures is again observed but it is displaced downwards to lower values. The degree of downward displacement increases with increasing Cd2+ concentration. Typical ionic crystals Alkaline earth fluoride The most important defect in this group is probably the anion Frenkel defect in which an interstitial F- ion occupies the center of a cubic that has eight F- ion at the corners. Conductivity measurements have shown that the anion vacancy is more mobile than the interstitial F- ion. This contrasts with AgCl in which the interstitial Ag+ is more mobile than the cation vacancy. In some materials that have the fluorite structure, e. g. PbF 2, the conductivity at high temperatures becomes quite large . At room temperature, PbF2 has very low ionic conductivity and as such is a typical ionic solids. With rising temperature its conductivity increases smoothly and rapidly until at 500 oC a limiting values of ~5(Ωcm)-1 is reached. 500oC 180oC Interstitial FFPb+2 Solid Electrolytes 500oC 200oC 27oC Solid electrolytes as intermediate between normal crystalline solids and liquids Ionic conductivity of some solid electrolytes with concentrated H2SO4 for comparison. Mobile ions in electrolytes CaF2 : F-; ZrO2 : O-2; AgI, RbAgI5 , AgCl : Ag+, Na3Zr2PSi2O12, Na:β-alumina : Na+ β- alumina β-alumina is the name for a family of compounds of general formula M2O.nX2O3 where n can have various values in the range 5 to 11, M is a monovalent cation, such as alkali ion, Cu+, Ag+, Tl+, NH4+, H3O+, and X is a thivalent cation, Al3+, Ga3+, or Fe3+. The most important member of this family is sodium β-alumina (M=Na+1, X=Al+3), which has been known for many years. The high conductivity of monovalent ions in βalumina is a consequence of its unusual crystal structure. The Na+ ions reside in the oxygen-deficient layers and are able to move very easily because (a) there are more sites available than there are Na+ ions to occupy them and (b) the radius of Na+ is less than that of the O2- ion. There are exists in two structural modifications, named β and β”, which differ in the stacking sequence of the layers. The β” forms with more soda-rich crystals, n~5-7, whereas β occurs for n~8-11. The conductivity of β” is several times larger than that of β. Conduction of some single crystal β-aluminas. Activation energies, in electronvolts, are in parentheses. Crystal structure of β- alumina Oxygen-deficient layer Oxide packing arrangement in β and β”-alumina oxide Oxide layer in β- alumina Na+ sb: spinel block Conduction plane in β-alumina C : O-2 in conduction plane A, B : O-2 in wall plane m : empty site br, abr, m : possible Na+ site Conduction pathway in β-alumina The conduction pathway of Na+ ion must through the sequence –br-m-abr-m-br-m-. The activation energy, which is 0.16ev, represents the overall value for migration of a Na + ion from one br to the next. Both knock-on and interstitialcy mechanism are possible. Na+ ions cannot move independently to each other in β–alumina bur that, instead, a cooperative process must occur. AgI and Ag+ ion solid electrolytes 146oC α –AgI β -AgI β-AgI stable below 146oC, has the wurtzite structure with hexagonal close packed I- ions and Ag+ in tetrahedral sites. The highly conducting α-AgI phase is body center cubic. In it, iodide ions lie at corner and body center positions and Ag+ ions appear to be distributed statistically over a total of thirty-six sites of tetrahedral and trigonal coordination. The iodide ions are essentially fixed and Ag+ ions can readily move from one site to the next in a liquid-like manner. The disordered Ag+ arrangements and the easy motion of Ag+ between sites must be related to the nature of the bonding between silver and iodine. Silver is a polarizing cat ion since its outer 4d electrons are relatively ineffective in shielding nuclear charge. Iodide is a 1arge and polarizable anion and so covalent bonds readily form between Ag+ and I- that are characterized by structures with low coordination numbers. During conduction, silver can readily move from one tetrahedral site to the next via an intermediate, three-coordinate site; covalent bonding at the intermediate site helps to stabilize it and reduce the activation energy for conduction. α -AgI The I- anions (shown blue) form a b.c.c. unit, with the Ag cations rapidly diffusing across distorted tetrahedral interstitial sites (shown red) through the trigonal interstitial sites (shown green). The black circles show distorted octahedral sites. β-AgI The phase diagram for the system Rb-Agl Two binary compounds are present, Rb2AgI3 and RbAg4I5. The latter melts incongruently to AgI and liquid at ~230oC and has a lower limit of stability at 27oC. Decomposition of RbAg4I5 to AgI and Rb2AgI3 below 27oC takes place only slowly although it is hastened by the presence of moisture or iodine vapor. With care, RbAg4I5 may be readily cooled to below room temperature without decomposition. In order to prepare RbAg4I5, a 1:4 molar mixture of RbI and AgI is melted in vacuum at ~500oC and then quenched to room temperature. On subsequent annealing at 165oc for 10hr this reacts further to give RbAg4I5. The crystal structure of RbAg4I5 is rather different to that of α-AgI, but it also contains a random arrangement of silver atoms distributed over a network of face-sharing tetrahedral sites. Rubidium atoms are immobilized in sites that have a distorted octahedral environment of I- ions. Sodium superionic conductor (NASICON, Na3Zr2PSi2O12) The framework is built of corner – sharing ZrO6 octahedra and (P, Si)O4 tetrahedra and a three-dimension network of tunnels in which Na+ ions reside. Oxide ion conductors The high temperature, cubic polymorph of zirconia has the fluorite crystal structure, and may be stabilized to room temperature by formation of solid solution with CaO, Y2O3, etc. Such stabilized zirconia are good O2- ion conductors at high temperatures, mainly because the mechanism of so1id solution formation involves the creation of vacant O2- sites in order to preserve electric neutrality, e.g. lime-stabilized zirconia has the formula (CaxZr1-x)O2-x: 0.1≦x≦0.2. With each Ca2+ ion that is introduced one O2- ion vacancy is created. Typical conductivities in stabilized zirconias (e. g. 85mol%ZrO2, 15mol%CaO) are 5×10-2 (Ω-cm)-1 at 1000oC With an activation energy 1.3ev. At lower temperatures stabilized zirconias have conductivities that are many order of magnitude 1ess than those of the Na+ and Ag+ ion solid electrolytes. Their usefulness stems from the fact that they are refractory materials, can be used up to very high temperatures (e. g. 1500oC) and have good oxide ion conductivity, which is an unusual property. Thoria (ThO2) and hafnia (HfO2) may be doped and are good O2- ion conductors at high temperatures. 1250oC 600oC Glasses ion conductor Alkali borate glasses Alkali silicate glasses Phosphate glasses The best we can do at present is to have a set of guidelines which show us the likely structural characteristics that are a prerequisite for high ionic conductivity. These are (a) A large number of the ions of one species should be mobile (i.e. σ=neμ) (b) There should be a large number of empty sites available for the mobile ions to jump into. This is essentially a corollary of (a) because ions can be mobile only if there are empty sites available for them to occupy. (c) The empty and occupied sites should have similar potential energies with a low activation energy barrier for jumping between neighboring sites. It is no use having a large number of available interstitial sites if the moving ion cannot get into them. (d) The structure should have a framework, preferably three dimensional, permeated by open channels through which mobile ions may migrate. (e) The anion framework should be highly polarizable. 研究方法 結構: X光繞射,IR,Raman,NMR 導電性質與機制: 交流阻抗分析,NMR 導電性質與機制: 交流阻抗分析 (RLi2O-B2O3 glasses) τhopping=τoexp(Ep/kT) 導電機制: NMR (ΔH1/2)2=(2/ π) (ΔH(0)1/2)2tan-1(α τnmrΔH1/2) A : WP= β T2 (phono interaction) B : WD= α exp(-ET1/kT) (diffusion)