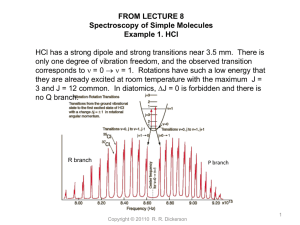
Virtual Temperature: Tv or T* - Atmospheric and Oceanic Science
advertisement

AOSC 620 Lecture 2 PHYSICS AND CHEMISTRY OF THE ATMOSPHERE I Professor Russell Dickerson Room 2413, Computer & Space Sciences Building Phone(301) 405-5391 russ@atmos.umd.edu web site www.meto.umd.edu/~russ Copyright © 2014 R. R. Dickerson 1 Experiment: Room temperature What is the temperature of the room? We’ll get 22 answers. How well do they agree? Does location matter? Does observer bias play a role? Copyright © 2013 R. R. Dickerson & Z.Q. Li 2 Experiment: Room temperature Last class Average oC Stdev oC Max oC Min oC TMean = +/- °C (+/- °F) Measured with uncalibrated or no thermometers at n locations. Copyright © 2010 R. R. Dickerson & Z.Q. Li 3 What is the right answer? What is the uncertainty? Are the data Gaussian? How can we improve the measurement? Copyright © 2013 R. R. Dickerson & Z.Q. Li 4 Experiment: Room temperature Let’s repeat the experiment with calibrated thermometers. Copyright © 2013 R. R. Dickerson & Z.Q. Li 5 Lecture 2. Thermodynamics of Air, continued – water vapor. Objective: To find some useful relationships among air temperature, volume, and pressure. Review Ideal Gas Law: PV = nRT Pα = R’T First Law of Thermodynamics: đq = du + đw W = ∫ pdα Copyright © 2013 R. R. Dickerson & Z.Q. Li 6 Review (cont.) Definition of heat capacity: cv = du/dT = Δu/ΔT cp = cv + R Reformulation of first law for unit mass of an ideal gas: đq = cvdT + pdα đq = cpdT − αdp Copyright © 2013 R. R. Dickerson & Z.Q. Li 7 Review (cont.) For an isobaric process: đq = cpdT For an isothermal process: đq = − αdp = pdα = đw For an isosteric process: đq = cvdT = du For an adiabatic process: cvdT = − pdα and cpdT = αdp Copyright © 2013 R. R. Dickerson & Z.Q. Li 8 Review (cont.) For an adiabatic process: cvdT = − pdα and cpdT = αdp du = đw (T/T0) = (p/p0)K Where K = R’/cp = 0.286 (T/θ) = (p/1000)K Define potential temperature: θ = T(1000/p)K • Potential temperature, θ, is a conserved quantity in an adiabatic process. Copyright © 2013 R. R. Dickerson & Z.Q. Li 9 Review (cont.) The Second Law of Thermodynamics is the definition of φ as entropy. dφ ≡ đq/T ჶ dφ = 0 Entropy is a state variable. Δφ = cpln(θ/θ0) In a dry, adiabatic process potential temperature doesn’t change, thus entropy is conserved. Copyright © 2013 R. R. Dickerson & Z.Q. Li 10 Useful idea - a perfect or exact differential: If z = f(x,y), dz is a perfect differential iff: ∂2f/∂x∂y = ∂2f/∂y∂x ჶ dz = 0 For example, v = f(T,p) dv = (∂v/∂p)T dp + (∂v/∂T)p dT This is true for dU, dH, dG, but not đw or đq. Copyright © 2013 R. R. Dickerson & Z.Q. Li 11 Various Measures of Water Vapor Content • Vapor pressure • Vapor density – absolute humidity • Mixing ratio, w (g/kg) • Specific humidity • Relative humidity • Virtual temperature (density temp) • Dew point temperature • Wet bulb temperature • Equivalent temperature • Isentropic Condensation Temperature •Potential temperature •Wet-bulb potential temperature •Equivalent potential temperature Copyright © 2013 R. R. Dickerson & Z.Q. Li 12 Virtual Temperature: Tv or * T Temperature dry air would have if it had the same density as a sample of moist air at the same pressure. Question: should the virtual temperature be higher or lower than the actual temperature? Copyright © 2013 R. R. Dickerson & Z.Q. Li 13 Consider a mixture of dry air and water vapor. Let Md = mass of dry air Mv = mass of water vapor md = molecular weight of dry air mv = molecular weight of water. Copyright © 2013 R. R. Dickerson & Z.Q. Li 14 Dalton’s law: P = Spi * * R R P pd e d T v T md mv Md Mv md mv M Md Mv V V * RT P V (V volum e) Copyright © 2013 R. R. Dickerson & Z.Q. Li 15 Combine P and to eliminate V: Md Mv 1 P R T md mv M d M v w 1 * 1 R T md mv 1 w * * R 1 T (1 w / ) md 1 w (1 w / ) P RT (1 w) Copyright © 2013 R. R. Dickerson & Z.Q. Li 16 Since P = RT* (1 w / ) and P RT (1 w) (1+ w / e ) T = virtual temperature = T (1+ w) * T > T because e = 0.622 * Copyright © 2013 R. R. Dickerson & Z.Q. Li 17 Alternate derivation: Since proportional to Mwt (md ) T virtual tem perature T (mw) md 29g / m ole * mw 29(1 x ) 18x Where w is the mass mixing ratio and x (molar or volume mixing ratio) = [H2O] = w/0.62 T* = T (29/(29-11[H2O])) e.g., [H2O] = 1% then T* = T(1.004) If , [H2O] = 1% then w = 0.01*.62 = 0.062 T* = 1.01/1.0062 = Copyright © 2013 R. R. Dickerson & Z.Q. Li 18 Where w is the mass mixing ratio and x (molar or volume mixing ratio) = [H2O] = w/0.62 T* = T (29/(29-11[H2O])) e.g., [H2O] = 1% then T* = T(1.004) Test: if [H2O] = 1% then w(18/29) = 0.01*.62 = 0.0062 T* = T (1 + w/)/(1+w) = T (1 + 0.01)/(1+0.0062) = T(1.004) Copyright © 2013 R. R. Dickerson & Z.Q. Li 19 Unsaturated Moist Air Equation of state: Pa = RT* (1+ w / e ) T =T (1+ w) (1+ w / e ) Pa = R T = RmT (1+ w) (1+ w / e ) where Rm = R @ R(1+ 0.6w) (1+ w) * because w £ 10 -1 so (1+ w)-1 @ 1- w Copyright © 2013 R. R. Dickerson & Z.Q. Li 20 Specific Heats for Moist Air Let mv = mass of water vapor md = mass of dry air To find the heat flow at constant volume: d Q (mv md ) d q md cv dT mv cvv dT mv mv cvv md cv dT 1 md 1 md md c v (1 w) d q cv dT (1 rw) with r cvv cv 1.96 Copyright © 2013 R. R. Dickerson & Z.Q. Li 21 For constant pressure c pm c p (1 0.9w) So Poisson’s equation becomes k 1000m b T P Rm R k (1 0.2 w) c pm c p (1+0.6w)/(1+0.9w) => (1-0.2w) due to rounding error. Copyright © 2013 R. R. Dickerson & Z.Q. Li 22 Water Vapor Pressure Equation of state for water vapor: ev = v Rv T where ev is the partial pressure of water vapor R* Rv mv R * md R * md eva RvT T T mv md md mv RdT / mv 0.622 md Copyright © 2013 R. R. Dickerson & Z.Q. Li 23