Lecture #13 Carbon Monoxide
advertisement

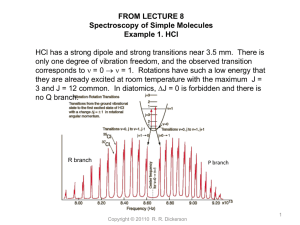
Carbon Monoxide Lecture AOSC 637 Atmospheric Chemistry Russell R. Dickerson Finlayson-Pitts Chapt. 16 Seinfeld Chapt. 2 & 6 Wallace & Hobbs Chapt. 5 EPA 2000 Criteria Document: http://www.epa.gov/NCEA/pdfs/coaqcd.pdf EPA 2010 ISA: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=218686 OUTLINE Importance Detection Techniques Sources and Sinks Global Chemistry & Trends Remaining Challenges References Copyright © 2010 R.R. Dickerson 1 Carbon Monoxide Importance • Primary Air Pollutant • Major sink for OH (greenhouse forcing, esp. short term!) Thompson et al. (1989); Shindell et al. (2009); Hoor et al. (2009) • Source/Sink of O3 depending on NOx • Toxic air pollutant Esp. for individuals with Coronary Artery Disease (EPA 2010) • Excellent tracer for combustion and dynamics. Copyright © 2010 R.R. Dickerson 2 Copyright © 2010 R.R. Dickerson 3 Copyright © 2010 R.R. Dickerson 4 In the remote atmosphere there is often insufficient NOx to drive this reaction to two O3; the process reduces OH. Globally, Thompson et al. (1989) predict that increased CO increases H2O2 and the ratio of HO2 to OH, but reduces OH. Reduced OH means a longer lifetime for CH4 and O3 which contribute to global warming, e.g., Shindell et al., (2009); EPA (2010) Copyright © 2010 R.R. Dickerson 5 Chemistry Carbon monoxide oxidation in a clean environment: (1) O3 + h O2 + O(1D) (2) O(1D) + H2O 2OH (3) OH + O3 HO2 + O2 (4) HO2 + O3 2O2 + OH ----------------------------------------(3+4) 2O3 3O2 NET Copyright © 2010 R.R. Dickerson 6 Chemistry, continued Carbon monoxide oxidation in a dirty (polluted) environment: (3') OH + CO H + CO2 (4') H + O2 + M HO2 + M (5') HO2 + NO NO2 + OH (6') NO2 + h NO + O (7') O + O2 + M O3 + M ------------------------------------------------Dickerson+ O (3'-7') CO Copyright + 2 O©22010R.R.CO 2 3 NET 7 Detection Methods • GC-FID • Hg Liberation (CO + HgO → CO2 + Hg↑) • Gas Filter Correlation NDIR • FTIR • Fluorescence • Tunable Diode Laser Spectroscopy • Remote sensing NDIR Copyright © 2010 R.R. Dickerson 8 Non-Dispersive Gas-Filter Correlation Detection of Carbon Monoxide Copyright © 2010 R.R. Dickerson 9 Copyright © 2010 R.R. Dickerson 10 Tunable Diode Laser Spectroscopy: Schematic Diagram Copyright © 2010 R.R. Dickerson 11 A TDL can be finely tuned to the precise wavelength that characterizes whatever chemical its users wish to detect. By measuring how much light has been absorbed, the TDL-based detector can determine how much carbon monoxide is present. The laser is tuned on and off a single rotational line around 4.6 mm to generate an AC signal. The signal is most easily seen as the second derivative. Copyright © 2010 R.R. Dickerson 12 MOPITT (Measurement of Pollution in the Troposphere): MOPITT is the first satellite sensor to use gas correlation spectroscopy. GCS is a non-dispersive technique to increase the sensitivity of the instrument to the gas of interest by separating out the regions of the spectrum where the gas has absorption lines and integrating the signal from just those regions. The specific wavelengths are located using a sample of the gas as a reference for the spectrum. By using correlation cells of differing pressures, some height resolution can be obtained. Copyright © 2010 R.R. Dickerson 13 MOPITT (Measurement of Pollution in the Troposphere) http://terra.nasa.gov/ Copyright © 2010 R.R. Dickerson 14 MOPITT CO image from EPA ISA. Copyright © 2010 R.R. Dickerson 15 Geostationary Remote Infrared Pollution Sounder [GRIPS] Science Overview GRIPS will measure columns of CO2, CH4, and CO with high accuracy and sensitivity down to the surface. From geostationary orbit over Asia where no wide-scale monitoring of these key pollutants and greenhouse gases currently exists we will determine sources and fluxes. Asia is a large but uncertain source CO as seen from Geo at 120oE of these gases. CO: Toxic. Emissions of pollutants such as NOx, SO2, and aerosols can be estimated from ratios to CO. Plumes from Asia travel across the Pacific and have large-scale adverse impacts. CH4: A major greenhouse gas that affects the oxidizing capacity of the atmosphere and tropospheric ozone CO2: The key greenhouse gas; Asian sources large and changing rapidly. Key to understanding fluxes will the the ratios of trace gas species which provide unique fingerprints. GRIPS can distinguish sources of pollution using ratios of CO2/CO and Power Generation Biomass Vehicles CO2/CH4. Industrial and burning residential sources can be further distinguished using data from a GRIPS UV/Vis spectrometer such as alone Estimated CO2 Source Regions GEMS that measures SO2, NO2, CO/BC* and Black Carbon. CO/NO2 E+02 E+01 E+00 CO2/CO E+03 CO2/CH4 E+04 CO2/CO CO2/CH4 for Power GeneraFingerprints on Vehicles Major Chinese Emission Sources Biomass Burning E+03 E+02 CO/SO2 0E+01 E+00 SO2/NO2 0E-01 0E-02 Power Genera on Industry Industry Residential Residen al Vehicles Biomass Burning GRIPS + GEMS * Black Carbon Total PI: Prof. Russell Dickerson U of MD russ@atmos.umd.edu Geostationary Remote Infrared Pollution Sounder [GRIPS] Instrument Overview GRIPS uses the well-understood gas filter correlation radiometry (GFCR) technique. GFCR, used by a number of satellite instruments including HALOE and MOPITT, employs tubular cells containing the target gas as a filter. These eliminate the need for a dispersive element, while providing outstanding spectral resolution, resulting in column concentrations of CO2, CH4, CO with sensitivity down to the surface and some altitude resolution in the troposphere. Our GFCR will use reflected solar IR for CO2 and CH4 and both solar and thermal IR for CO. Nadir pixel size is ~4x4 km. Species Instrument 30 cm Bundle 20 cm Wavelength (µm) CO2 2.05 - CH4 2.28 - CO 2.33 4.64 The GRIPS Instrument Mass: 13 kg Power: 15W Data Rate: 6.5 Gbits/day Volume: 0.012 m3 Nadir pixel: 8 km2 Continental scan: 1 hour Disk scan: 2 hours 20 cm To measure each species, light is brought through a 4 telescope bundle hosting gas cells at different pressures. Light is then concentrated to a 4 partition cooled MerCadTel detector using a pyramidal mirror. In the instrument configuration shown, one bundle is for CH 4, one for CO2 CO/NOX CO2/CO CO2/CH4 CO/SO2 Sources Natural: Methane oxidation. Biogenic hydrocarbon (esp. isoprene) oxidation. Direct emission from plants and oceans, although plants may absorb CO as well as emit it. In any case, only direct emission is small relative to HC oxidation. Anthropogenic: Internal combustion engines emit CO, especially when they run rich. Even at a stoichiometric air/fuel mixture, CO is produced because of hightemperature dissociation of CO2. CO2 → CO + ½O2 CO + ½O2 → CO2 H = -67.6 Coal combustion does not generate much CO because the power plants run lean in order to extract as much energy from the coal as possible. Biomass burning is a major source, as is oxidation of anthropogenic hydrocarbons in the presence of NOx. Copyright © 2010 R.R. Dickerson 19 American CO Emissions 2008 MISCELLANEOUS 17% c OFF-HIGHWAY 26% HIGHWAY VEHICLES 57% Direct anthropogenic emissions only, based on the Copyright © 2010 R.R. Dickerson Mobile6 model. (EPA, 2009) 20 Trend in American CO Emissions Thousands of tons per year 250000 200000 150000 100000 50000 0 1965 1970 1975 1980 1985 1990 1995 2000 2005 2010 Year Copyright © 2010 R.R. Dickerson 21 Copyright © 2010 R.R. Dickerson 22 Comparison of observed (jagged red line) and modeled morning CO vertical profiles paired in time and space over Mid Atlantic region. The solid lines are the medians, and the shaded areas represent the 25th and 75th quartiles of the data. The blue line corresponds to modeled CO with the deposition velocity calculated in MCIP v3.4.1, and the green line corresponds to modeled CO with the deposition velocity set to zero. The total CO column below 1000 m is closer to observations when the CO deposition velocity is set to zero, but the shape of the vertical profile does not change substantially (Castellanos et al., 2010). Copyright © 2010 R.R. Dickerson 23 Copyright © 2010 . Same as previous figure except comparison of observed and modeled afternoon CO vertical profiles. The top graph corresponds to observations of poorly mixed air near the surface (51 profiles). The bottom graph corresponds to observations of well mixed air near the surface (21 profiles). The local maximum observed around 2000 m cannot be produced by diffusive processes, but may be generated by smallscale convective processes such as those associated with fair weather cumulus clouds. R.R. Dickerson 24 25 Model Performance of CO in CMAQ Turbulent transport is modeled with an eddy diffusion coefficient (Kz, m2/s) ci t VDIFF ci Kz z z Analogous to molecular diffusivity K Turbulent z may be overestimated mixing Copyright © 2010 R.R. Dickerson 26 Model Performance of CO in CMAQ Convective Transport Afternoon Well Mixed Asymmetric Convective Model Copyright © 2010 R.R. Dickerson 27 Model Performance of CO in CMAQ Convective Transport Underestimated Afternoon Poorly Mixed Fast turbulent mixing near the surface Copyright © 2010 R.R. Dickerson High resolution WRF-UCM and CMAQ modeling Christopher P. Loughner1, Dale J. Allen1, Russell R. Dickerson1, Kenneth E. Pickering1,2 Yi-Xuan Shou3, and Da-Lin Zhang1 1Department of Atmospheric and Oceanic Science, University of Maryland, College Park, MD 2NASA Goddard Space Flight Center, Greenbelt, MD 3National Satellite Meteorological Center, China Meteorological Administration, Beijing, China MDE Quarterly Meeting April 25, 2011 Introduction • Investigate the influence of grid resolution on the Chesapeake Bay breeze, the dispersion of pollutants, and ozone formation. • The Chesapeake Bay breeze influences: – – – – Horizontal advection; Convergence; Stagnation; and Vertical transport WRF-UCM 2-m temperature and 10-m wind speed at 1200 UTC (7 am EST) July 9, 2007. Westerly winds in the 13.5 and 0.5 km simulations transporting near surface pollutants over the Chesapeake Bay. WRF-UCM 2-m temperature and 10-m wind speed at 1400 UTC (9 am EST) July 9, 2007. Stagnation in northern end of Chesapeake Bay in the 0.5 km simulation causes pollutants to accumulate. WRF-UCM 2-m temperature and 10-m wind speed at 1900 UTC (2 pm EST) July 9, 2007. A stronger temperature gradient along the coastline of the Chesapeake Bay in the 0.5km domain results in a stronger Bay breeze. Ozone concentrations at 1900 UTC (2 pm EST) July 9, 2007. Early morning stagnation over the Chesapeake Bay allowed high ozone concentrations to be transported northward in the higher resolution simulations. Cross-section of CO between Washington, DC and Baltimore, MD for the 13.5 and 0.5km simulations. The stronger bay breeze in the 0.5km simulation causes higher pollutant concentrations at the bay breeze convergence zone where they are lofted and then transported downwind. coastline 13.5km coastline 0.5km Profiles near western shore coastline CO O3 Edgewood profiles Edgewood profiles Edgewood profiles Edgewood profiles Edgewood profiles Edgewood profiles Edgewood profiles Conclusions • The Chesapeake Bay breeze causes: – Stagnation and accumulation of pollutants in the bay; – Convergence of pollutants at the bay breeze convergence zone; and – Lofting of pollutants at the bay breeze convergence zone. The global distribution of CO reflects the dominance of emissions in the Northern Hemisphere, the seasonal cycle of OH, and the short lifetime relative to transport across the ITCZ. Copyright © 2010 R.R. Dickerson 44 From the NOAA CMDL Trends Network Available almost real time. http://www.esrl.noaa.gov/gmd/ccgg/iadv/ Copyright © 2010 R.R. Dickerson 45 Copyright © 2010 R.R. Dickerson 46 Copyright © 2010 R.R. Dickerson 47 Copyright © 2010 R.R. Dickerson 48 Copyright © 2010 R.R. Dickerson 49 Remaining Challenges related to CO in the atmosphere How accurate are the emissions from Mobile6? Finlayson-Pitts, in Ch. 16 of her 2000 book, pointed out that emissions were a problem. Things do not seem much better today. Parrish (2006) “Mobile6 too high by factor of 2” Castellanos et al., (2010) “About right (+/-10%)” Yu et al., 2007, 2009 “20-30% low bias except NYC high bias” Marmur et al., (2009) “a 36% low bias” Kuhns et al. (2004) “factor of ~2 too high for gasoline-powered vehicles” Bishop and Stedman [2008] “deterioration rate of control technology in motor vehicles was overestimated by a factor of five” Hudman et al. [2008] and Warneke et al. [2006] “modeled CO anthropogenic emissions are 50-60% too high.” Copyright © 2010 R.R. Dickerson 50 CO/NOx ratio from observations indicates a ratio of ~6:1 (Luke et al., 2010). Emissions inventories (http://www.epa.gov/ttnchie1/trends/) indicate a ratio of 12:1. Copyright © 2010 R.R. Dickerson 51 References Bishop, G. A. and D. H. Stedman (2008), A decade of on-road emissions measurements, Environmental Science & Technology, 42, 1651-1656. Castellanos, P., L. T. Marufu, B. G. Doddridge, B. F. Taubman, S. H. Ehrman, and R. R. Dickerson (2010), Evaluation of Vertical Mixing and Emissions in the CMAQ Model Using Measured Vertical Profiles of CO and O3, J. Geophys. Res., in preparation. Hoor, P., J. Borken-Kleefeld, D. Caro, O. Dessens, O. Endresen, M. Gauss, V. Grewe, D. Hauglustaine, I. S. A. Isaksen, P. Jockel, J. Lelieveld, G. Myhre, E. Meijer, D. Olivie, M. Prather, C. S. Poberaj, K. P. Shine, J. Staehelin, Q. Tang, J. van Aardenne, P. van Velthoven, and R. Sausen (2009), The impact of traffic emissions on atmospheric ozone and OH: results from QUANTIFY, Atmospheric Chemistry and Physics, 9, 3113-3136. Hudman, R. C., L. T. Murray, D. J. Jacob, D. B. Millet, S. Turquety, S. Wu, D. R. Blake, A. H. Goldstein, J. Holloway, and G. W. Sachse (2008), Biogenic versus anthropogenic sources of CO in the United States, Geophysical Research Letters, 35. Hudman, R. C., L. T. Murray, D. J. Jacob, S. Turquety, S. Wu, D. B. Millet, M. Avery, A. H. Goldstein, and J. Holloway (2009), North American influence on tropospheric ozone and the effects of recent emission reductions: Constraints from ICARTT observations, Journal of Geophysical Research-Atmospheres, 114, DOI: 10.1029/2008JD010126 Kuhns, H. D., C. Mazzoleni, H. Moosmuller, D. Nikolic, R. E. Keislar, P. W. Barber, Z. Li, V. Etyemezian, and J. G. Watson (2004), Remote sensing of PM, NO, CO and HC emission factors for on-road gasoline and diesel engine vehicles in Las Vegas, NV, Science of the Total Environment, 322, 123-137, DOI: 10.1016/j.scitotenv.2003.09.013 Luke, W. T., P. Kelley, B. L. Lefer, and M. Buhr (2010), Measurements of primary trace gases and NOy composition in Houston, Texas, Atmospheric Environment, in press, DOI: 10.1016/j.atmosenv.2009.08.014. Marmur, A., W. Liu, Y. Wang, A. G. Russell, and E. S. Edgerton (2009), Evaluation of model simulated atmospheric constituents with observations in the factor projected space: CMAQ simulations of SEARCH measurements, Atmospheric Environment, 43, 1839-1849, DOI: 10.1016/j.atmosenv.2008.12.027. Novelli, P. C., K. A. Masarie, P. M. Lang, B. D. Hall, R. C. Myers, and J. W. Elkins (2003), Reanalysis of tropospheric CO trends: Effects of the 1997-1998 wildfires, Journal of Geophysical Research-Atmospheres, 108. Parrish, D. D. (2006), Critical evaluation of US on-road vehicle emission inventories, Atmospheric Environment, 40, 2288-2300. Shindell, D. T., G. Faluvegi, D. M. Koch, G. A. Schmidt, N. Unger, and S. E. Bauer (2009), Improved Attribution of Climate Forcing to Emissions, Science, 326, 716-718. Thompson, A. M., R. W. Steward, M. A. Owens, and J. A. Herwehe (1989), Sensitivity of tropospheric oxidants to global chemical and climate change, Atmos. Environ., 23, 519-532. Warneke, C., J. A. de Gouw, A. Stohl, O. R. Cooper, P. D. Goldan, W. C. Kuster, J. S. Holloway, E. J. Williams, B. M. Lerner, S. A. McKeen, M. Trainer, F. C. Fehsenfeld, E. L. Atlas, S. G. Donnelly, V. Stroud, A. Lueb, and S. Kato (2006), Biomass burning and anthropogenic sources of CO over New England in the summer 2004, Journal of Geophysical Research-Atmospheres, 111, DOI: 10.1029/2005JD006878. Yu, S. C., R. Mathur, D. W. Kang, K. Schere, and D. Tong (2009), A study of the ozone formation by ensemble back trajectoryprocess analysis using the Eta-CMAQ forecast model over the northeastern US during the 2004 ICARTT period, Atmospheric Environment, 43, 355-363. Yu, S. C., R. Mathur, K. Schere, D. W. Kang, J. Pleim, and T. L. Otte (2007), A detailed evaluation of the Eta-CMAQ forecast model performance for O-3, its related precursors, and meteorological parameters during the 2004 ICARTT study, Journal of Geophysical Research-Atmospheres, Copyright 112. © 2010 R.R. Dickerson 52 Take Home Messages • Carbon Monoxide is a relatively well understood trace gas with important roles in human health, the oxidizing capacity of the atmosphere and in global climate. • CO is a useful tracer for dynamical processed in the atmosphere such as convective mixing. • The uncertainty in the emissions is larger than can be explained by measurement uncertainty. Copyright © 2010 R.R. Dickerson 53