Young-Laplace & Kelvin Equations: Surface Chemistry
advertisement
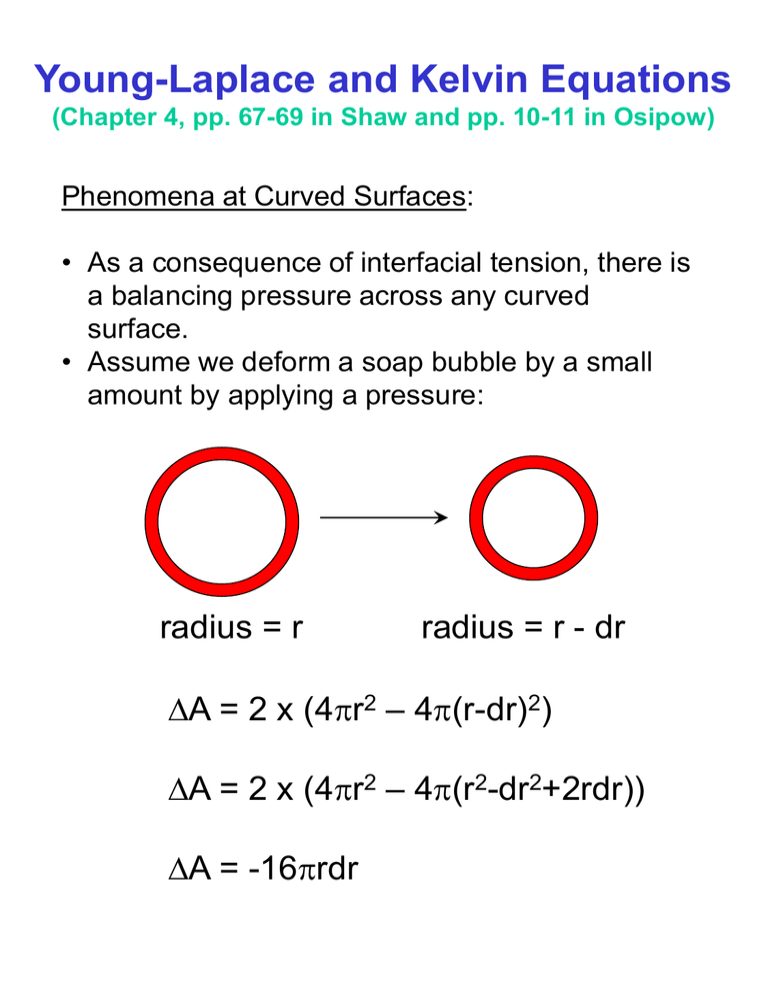
Young-Laplace and Kelvin Equations (Chapter 4, pp. 67-69 in Shaw and pp. 10-11 in Osipow) Phenomena at Curved Surfaces: • As a consequence of interfacial tension, there is a balancing pressure across any curved surface. • Assume we deform a soap bubble by a small amount by applying a pressure: radius = r radius = r - dr DA = 2 x (4pr2 – 4p(r-dr)2) DA = 2 x (4pr2 – 4p(r2-dr2+2rdr)) DA = -16prdr Two types of work are done: • Surface Energy Work DWS= 16 pgrdr • Pressure Work DWP= DP x 4pr2dr When the two are equal we get for the pressure across the curved surface: DP x 4pr2dr = 16pgrdr Hence: DP = 4g/r Known as the Young-Laplace equation for a hollow bubble. Other curved surfaces: Young-Laplace equation for a spherical droplet: DP = 2g/r Young-Laplace equation for any curved surface with principle radii of curvature r1 and r2: DP = g(1/r1 + 1/r2) 1/r2~0 1/r1 DP = g/r1 The Young-Laplace equation has been experimentally validated in the following ways: 1) Two connected soap bubbles. 2) Measurement of capillary rise for liquids with known surface tensions. h 3) Through a validation of the Kelvin equation which is derived from the Young-Laplace equation. The Kelvin Equation (Chapter 4, pp. 67-69 in Shaw) If the radius of a droplet increases from r to r+dr, the increase in surface free energy will be: DGi = 8pgrdr Pr Pr+dr Po If the process involves the transfer of dn moles of liquid from the plane surface with vapor pressure Po to the droplet with vapor pressure Pr, the free energy increase is also equal to: dn RT ln(Pr/Po) Thus: dn RT ln(Pr/Po) = 8pgrdr And: dn = 4pr2 dr r/M Then: Pr 2gM 2gVm RT ln Po rr r r is the density of the fuid; M is the molar mass of the fluid; and Vm is the molar volume. The above relationship is known as the Kelvin equation for liquid droplets. For water droplets: r, nm Pr/Po 100 10 1 1.01 1.1 3.0 The Kelvin equation has been experimentally verified with crossed mica cylinders (see Heimenz, p. 263): Pr For bubbles in a liquid the Kelvin equation is given as: Pr 2gM 2gVm RT ln Po rr r When the curvature is negative (i.e. concave) then a lowering of the vapor pressure is predicted. The Kelvin equation can easily explain the following processes: • Vapor condensing in small capillaries • Capillary rise phenomena • Supersaturation phenomena (scratching organic chemists) • Emulsion breakdown mechanism known as Ostwald ripening Next lectures: Measurement of Surface Tensions and Surfactants