Measurements and Density
advertisement

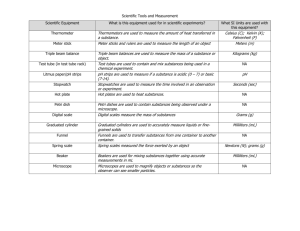
Scientific Measurements and Density Measurements are an everyday part of science class. It is very important to be as accurate as possible when taking measurements. Length In science, we use the metric system. So lengths are measured in units like millimeters (mm), centimeters (cm), meters (m), and kilometers (km). Take a look at the meter stick above. On the bottom, the longest lines represent each whole centimeter (cm). The medium length lines are each ½ or .5 of a centimeter (cm) or 5 millimeters (mm). The smallest lines are each 1 millimeter. There are 10 mm in 1 cm and there are 100 cm in 1 meter (m). Let’s figure out the answers now! A. 2.3 cm B. 5.1 cm C. 10.0 cm What is the distance between points… A and B: 2.8 cm A and C: 7.7 cm B and C: 4.9 cm Why is it better to align the meter stick so that the edge of the object being measured is at the 1 cm mark rather than the end to the meter stick? Because the meter stick may be worn at the edge and you will not get an accurate reading. But be careful!! If you start at the 1 cm mark, you must allow for that in your final answer by subtracting 1 cm from your answer. Length In making a measurement of length, the meter stick should be placed on its edge so that the scale rests on the object as shown on your paper. Can you explain why? Being eye-level will make your answer more accurate! Length An enlarged section of a metric ruler is shown below. There are letters along the bottom edge of the ruler. Write the correct measurement for each letter in the space next to the correct letter on your paper. A. 20.5 cm B. 19.3 cm C. 21.25 cm D. 21.95 cm E. 18.3 cm F. 20.0 cm G. 17.7 cm H. 20.95 cm I. 22.3 cm J. 19.7 cm Temperature Temperature is a measure of the average vibration of the particles that make up a substance. In other words, the faster the particles are vibrating, the warmer they are and thus the higher the temperature. BE CAREFUL! Temperature and heat are NOT the same thing!! Heat is a type of energy, temperature is a measure of that energy. Temperature The instrument used to measure temperature is a thermometer. The units of temperature are degrees. There are three different degree scales. 1. Fahrenheit (°F) – used in U.S. 2. Celsius (°C) - metric 3. Kelvin (K) – used mostly in science, known as the SI unit for temperature. The Kelvin scale is based on absolute zero. Temperature and your ESRT: pg. 13 Time In this class, you will be using a stopwatch most often to measure time. It’s VERY important to know how to read one accurately! PAY ATTENTION – this is not in your notes, just watch and learn! Stopwatches are generally divided into 4 sections: Hours, minutes, seconds, and milliseconds. In stopwatch “A” the correct reading would be 4.06 seconds. In stopwatch “B” the correct reading would be 6.59 seconds. What would be the readings of stopwatch A and B if you were asked to round to the nearest tenth? (one place after the decimal) A: 4.1 seconds A B: 6.6 seconds B Volume Volume is how much space an object takes up. Both liquids and solids have a volume. Volume of Liquids The instrument used to measure volume of liquids is a graduated cylinder. Most of the time, the units used to measure volume are milliliters (mL). Volume of Liquids meniscus 37.0 8.6 22.0 Volume of Rectangular Solids The most common instruments for measuring the volume of rectangular solids are rulers and/or meter sticks. The formula for volume of a rectangular solid is: V = L x W x H. Units are “cubic” units because there are three sides being multiplied by each other. cu. cm or cm³ cu. m. or m³ 1 cubic centimeter = 1 milliliter Let’s try it now! – determine the volume of each illustration on your paper. V=LxWxH V=LxWxH V=LxWxH V = (3cm)(3cm)(3cm) V = (8m)(6m)(3m) V = (4cm)(4cm)(10cm) V = 27.0 cm³ V = 144.0 m³ V = 160.0 cm³ Volume by displacement: used for solids that are irregularly shaped. 20.0 mL 25.0 mL 5.0 mL (25 mL – 20 mL) meniscus Mass Mass - how much matter or “stuff” is in an object. Traditionally, the instrument used to measure mass is a triple beam balance. However, in these “modern” times, we now use an electronic balance. The 2 units most commonly used to measure mass are grams (g) and kilograms (kg). This year you will be using an electronic balance like below. Basically, turn balance on, press the “zero” button, place object on the scale and it will automatically give you a reading! Weight Weight is the amount of gravitational force acting on an object. Mass and weight are NOT the same thing!!! Weight is a FORCE, Mass is the “stuff” in an object. The instrument used to measure weight is a spring scale. The metric units of weight are Newtons (N). The English units of weight are pounds (lb). Weight There are 2 factors that affect weight: 1. The amount of mass the object possesses. 2. The amount of gravity acting on the object. Weight Using a spring scale, the weight of this ruler can be measured. Another object, which is smaller, has less mass and so it weighs less. Weight The ruler, here on Earth, has a certain weight. However, on the moon, it weights much less due to less gravity pushing down on the ruler. Density Density is the amount of matter (stuff) that occupies a given space. In other words, it has to do with BOTH mass and volume. If an object has the same “stuff” in it, but gets bigger it will be less dense. If an object stays the same size, but has more “stuff” in it, it will be more dense. Density Least Dense Most dense Above, all three objects are the same size, but the one on the left has much less “stuff” in it than the one in the middle or the one on the right, so it is least dense. The one on the right has the most “stuff” (mass) inside it’s given volume, so it is most dense. Density The formula for density is: D = Mass/Volume The formula is found on page 1 of your ESRT. Using the density triangle, you can easily figure out the formulas for mass and volume as well. M D V Density Instruments: For density of liquids, you would use a graduated cylinder to get the volume and then an electronic balance for the mass. For density of solids, you would use a ruler and V=L x W x H to get the volume and an electronic balance for the mass. Density Units: It depends on what the object is and the units of mass and volume that were used. The two most common are: 1. g/mL 2. g/cm³ Notice that there are always TWO units in density because you are multiplying mass and volume and neither of those cancel each other out. Putting it all together now! Solving Density Problems Density = M / V D = 150 g / 10 cm³ V=LxWxH V = (5cm)(1cm)(2cm) V = 10 cm³ D = 15 g/cm³ Solving Density Problems D=M/V D = 125 g / 50 mL D = 2.5 g / mL 250 300 Object X 300 mL – 250 mL= 50 mL What if you already know the density of an object and asked to solve for mass or volume?? Use the density triangle! Example: The density of an object is 5.5 g/mL and the volume is 8 mL. What is the mass? 44.0 g Density Relationships There are several factors that may affect an object’s density. We will discuss four: Size Temperature State of Matter Pressure Density vs. Size SIZE DOES NOT MATTER!! – as long as the substance stays the same, the density will NOT change. Ex.) lead has the same density no matter if it’s a huge piece or a very small piece. Relationship Density Size Density vs. Temperature When an object heats up, the volume will increase, due to the object getting larger. The mass will stay the same and thus the density will decrease. Inverse or indirect relationship density Temp. Therefore, the relationship is as temperature increases density will decrease. Density vs. State of Matter solid liquid gas most least density Solid objects have a lot of “stuff” packed tightly together so, they are most dense. The particles in gases have a lot of room to move! •The “water rule” – the ONLY exception to this is that water is MOST dense in its LIQUID state at 4°C Water ONLY Regular Density Density S L G S L G Density vs. Pressure As more pressure is exerted on a gas, the volume will decrease, due to a smaller area for the particles to move. Now they are packed tightly together. The mass stays the same and therefore, the density will increase. Thus, the relationship is as pressure increases, density will also increase. Density Direct Relationship Pressure