Unit 19
advertisement

CHM 1046: General Chemistry and
Qualitative Analysis
Unit 19
Acid Base Equilibria:
Titrations
Dr. Jorge L. Alonso
Miami-Dade College –
Kendall Campus
Miami, FL
Textbook Reference:
•Chapter 19 (sec. 5-8)
•Module 9
Aqueous
Equilibria
•Soln-Unknown
Concentration (M): Acid
• Known
Volume (V)
Titration
•Standard-of known Conc.(M)
•Measure Vol to reach end pt.
MolesB = M x V
L
L
MolesA = M x V
A volumetric
technique in which
one can determine
the concentration
of a solute in a
solution of
unknown
concentration, by
making it react
with another
solution of known
concentration
(standard).
If: MolesA = MolesB
{*TitrationMovie}
Then: (M x V)A = (M x V)B
Aqueous
Equilibria
Determining the Concentration of
Solutions by Titration
A known concentration of
base (or acid) is slowly added to
a solution of acid (or base) until
neutralization occurs.
(Standard)
Example:
HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l)
xa HA (aq) + xb MOH (aq) MA (aq) + H2O (l)
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + H2O(l)
Neutralization: equivalence point
# mol1(acid) = # mol2(base)
1 mol1(acid) = 1 mol2(base)
xa
xb
1 H 2SO 4
? H2SO4 1NaOH Aqueous
2 NaOH
Equilibria
Titration Calculations:
Stoichiometry using Molarities
xA HN + xB MOH MN + HOH
1 moles(acid) = 1 moles(base)
xA
xB
Since moles = MV = moles x Liter
Liter
Neutralization:
# g
MW
xA
=
ηA
xA
Where
xA or B
=
MA x VA
xA
=
MB x VB
xB
=
ηB
=
xB
# g
MW
xB
= coefficients from balanced equations
* Equation Useful for determining Molarities and
Volumes at the Equivalence Point of a Titration *
Aqueous
Equilibria
Solution Stoichiometry Problems:
Molarity
Problem: A volume of 16.3 mL of a 0.30M NaOH solution was
used to titrate 25.00 mL H2SO4. What is the concentration of
H2SO4 in the solution of unknown concentration?
H2SO4 + 2 NaOH 2HOH + Na2SO4
MA x VA = MB x VB
x1A
1M B VB
MA
2 VA
xB
2
1 0.30M 16.3 mL
= 0.098 M H2SO4
2 25.00 mL
Titration of a Strong Acid with a Strong Base
Aqueous
Equilibria
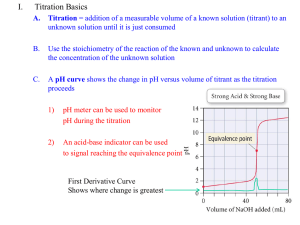
Titration Graph: pH vs. Volume
Titration
Data:
Excess base
mL of
NaOH
pH
0
1.2
10
1.4
20
1.6
30
1.7
40
1.9
50
7.0
60
12.0
pH
70
12.2
meter
80
12.3
SB
Phenolphthalein Indicator
Acid = Base
Methyl Red Indicator
Excess acid
SA
Aqueous
Equilibria
{Titration2}
Titrations: The Strength of
Acids & Bases
SA
Strong Base with Strong Acid
Weak Base with Strong Acid
SA
Phenolphthalein
8-10
SB
WB
Strong Base with Weak Acid
WA
SB
Weak Base with Weak Acid
WA
Aqueous
Equilibria
WB
(2) Titration of a WA with a SB
With weaker acids,
the initial pH is
higher and pH
changes near the
equivalence point
are more subtle.
Aqueous
Equilibria
Selecting Appropriate Indicators
Select appropriate indicator for following:
Phenolph
Meth Red
Phenolph
Meth Red
?????
• an indicator is chosen so that it will change color at a pH just beyond the
equivalence point (mid point of the steep vertical portion of the graph). The
first point at which the indicator permanently changes color marks the end of
the titration and is called the indicator end-point. Dropping a perpendicular
Aqueous
from the indicator end-point to the x-axis is a very close estimation ofEquilibria
the
equivalence point.
SB
SA
(1) Titration of a SA with a SB
(or SB with a SA)
SA
SB
Aqueous
Equilibria
Acid-Base Neutralization Equations
(1) Strong Acids and Bases are represented in completely dissociated state:
as H+ and OH-
(2) Weak Acids and Bases are represented in undissociated state:
as HA and B (or MOH)
Aqueous
Equilibria
(H2CO3 + K+)
(H2CO3 + Ca2+ + C2H3O2- )
(H2C O3 + Zn2+)
(H2CO3 + Zn2+ + SO42-)
Aqueous
Equilibria
Equations and Tables used in
solving A-B Titration Problems
MV A MV B
(1) Acid Base Neutralization: when you
reach the end-point using SA or SB
(2) Acid Base Neutralization: when not
at the end-point or using WA or WB
A
B
(3) WA or WB Equilibrium
problems
HA ↔ H+ + AHA + OH- H2O + A-
[ H ][ A ]
KA
[ HA ]
Use: Mole ICEnd Table
Mole
HA
(.15M)(.025L)
+
MOH
→
(.10M)(.030L)
MA
[ A ]
pH pKa log
[ HA]
Use: [ICE] Table
+ H20
HA
↔
H+
A-
II
0.0038 η
0.0030 η
0 η
II
0.061
0
C
- 0.0030 η
- 0.0030 η
+ 0.0030 η
C
-x
+x
Aqueous
Equilibria +x
0.0008 η
0 η
0.0030 η
E
0.061 - x
x
x
End
0
(1) Titration of a SA with a SB
Example: 25 mL of 0.15M HCl with 0.10M NaOH.
(1) What volume of NaOH is required to reach the equivalence point?
A B
A B
VB
MV A MV B
A
MV A
VB
B
B
A MB
0.15M .025L (1)
(1) 0.10M
37.5mL
(2) What is the pH of the initial strong acid? (strong acid problem)
In strong acid [HA]=[H+], so pH=-log [H+] = -log (0.15) = 0.82
Mole
(3) What is the pH prior to the equivalence point? Let’s say after 30. mL of
NaOH. (excess SA problem) *what is used for neutralization Rx?*
(0.0008)
HX
+
MOH
→
MX
+
H20
[ HX] [ H ]
(.15M)(.025L)
(.10M)(.030L)
L (.025L .030 L)
II
0.0038 η
0.0030 η
0
C
- 0.0030 η
- 0.0030 η
+ 0.0030 η
0.0008 η
0
0.0030 η
End
Salt of SA & SB:
will not Hydrolyze
[H ] 1.4 102 M
pH = -log (1.4 x 10-2) = 1.9
Aqueous
Equilibria
(1) Titration of a SA with a SB
Example: 25 mL of 0.15M HCl with 0.1M NaOH.
(4) What is the pH at the equivalence point?
Only salt + water present and salt will not hydrolyze water since it is
derived from SA & SB. So pH = 7
(5) What is the pH after the equivalence point? Lets say after 40. mL of
NaOH. (excess SB problem) *what is used for neutralization Rx?*
mole
HX
(.15M)(.025L)
MOH
+
→
MX
0.0038 η
0.0040 η
0 η
C
- 0.0038 η
- 0.0038 η
+ 0.0038 η
0.0002 η
0.0038 η
0η
[OH ]
H20
(.10M)(.040L)
II
End
+
0.0002
3.1103 M
(.025L .040L)
pH + pOH = pKw
Salt of SA & SB:
will not Hydrolyze
pOH = -log(3.1x10-3) = 2.5
pH = pKw - pOH
pH = 14 – 2.5 = 11.5
Aqueous
Equilibria
(2) Titration of a WA with a SB
SB (OH-)
HA ↔ H+ + AHA + OH- H2O + A*what is used for neutralization Rx?*
• Unlike in the previous
case, the conjugate base
of the acid affects the pH
when it is formed.
• The pH >7 at the
equivalence point.
*what is used for equilibrium Rx?*
[ H ][ A ]
KA
[ HA ]
[ A ]
pH pKa log
[ HA]
Aqueous
Equilibria
(2) Titration of a WA with a SB
Example: 25 mL of 0.15M HC2H3O2 (Ka= 1.8 X10-5) with 0.10M NaOH.
(1) What volume of NaOH is required to reach the equivalence point?
A B
A B
VB
MV A MV B
A
MV A
A MB
VB
B
B
0.15M 0.025L (1)
(1) 0.10M
37.5mL
(2) What is the initial pH of the acetic acid? (Before titration, WA
Equilibrium
problem)
HA
↔
H+
A[ H ][ A ]
K a
II
0.15
0
0
[ HA]
C
-x
+x
+x
1.8 105
( x)(x)
(0.15 x)
E
x 2 (1.8 105 )(0.15) 2.7 106
x [ H ] 0.0016
0.15 - x
x
x
pH log(0.0016) 2.8
Aqueous
Equilibria
(2) Titration of a WA with a SB
Example: 25 mL of 0.15M HC2H3O2 (Ka= 1.8 X10-5) with 0.10M NaOH.
(3) What is the pH prior to the equivalence point? Let’s say after 30. mL
of NaOH. (WA Buffer problem)
mole
HA
(.15M)(.025L)
→
MA
+
H20
(.10M)(.030L)
II
0.0038 η
0.0030 η
0 η
C
- 0.0030 η
- 0.0030 η
+ 0.0030 η
0.0008 η
0 η
End
[ HA]
MOH
+
(0.0008 )
0.015M
0.025L 0.030L
0.0030 η
[ A ]
Salt of WA & SB:
WILL Hydrolyze H2O
(0.0030 )
0.055M
0.025L 0.030L
[ A ]
pH pKa log
pKa log[ A ] log[HA]
[ HA]
Aqueous
Equilibria
pH log(1.8 105 ) log(0.055) log(0.015) (4.7) (1.3) (1.8) 5.2
(2) Titration of a WA with a SB
Example: 25 mL of 0.15M HC2H3O2 (Ka= 1.8 X10-5) with 0.1M NaOH.
(4) What is the pH at the equivalence point? This happens @ 37.5 mL
(Hydrolysis of Salt derived from a WA & SB)
mole
HA
(.15M)(.025L)
+
MOH
→
MA
H20
(.10M)(.038L)
II
0.0038 η
0.0038 η
0 η
C
- 0.0038 η
- 0.0038 η
+ 0.0038 η
0η
0 η
End
+
0.0038 η
Salt of WA & : will
Hydrolyze water
Salt is NaC2H3O2
Na+ = derived from SB (NaOH), will not hydrolyze.
C2H3O2- = derived from WA (acetic acid) it WILL hydrolyze water.
Aqueous
Equilibria
(2) Titration of a WA with a SB
Example: 25 mL of 0.15M HC2H3O2 (Ka= 1.8 X10-5) with 0.1M NaOH.
(4) What is the pH at the equivalence point? This happens @ 37.5 mL
(Hydrolysis of Salt derived from a WA & SB)
mole
HA
+
(.15M)(.025L)
MOH
→
MA
0.0038 η
0.0038 η
0 η
C
- 0.0038 η
- 0.0038 η
+ 0.0038 η
0η
0 η
0.0038 η
C2H3O2- + H2O
K w Ka K b
0.0038
0.066 M
(.025L .038L)
[C2H3O2-] =.
Salt is NaC2H3O2
↔
HC2H3O2
+
OH-
II
0.061
0
0
C
-x
+x
+x
E
0.061 - x
x
x
K w 1.0 1014
10
K b
5
.
6
10
K a 1.8 105
pH + pOH = pKw
H20
(.10M)(.038L)
II
End
+
[ HA][OH ]
( x)(x)
10
K b
5.6 10
(0.061 x)
[A ]
x 2 (5.6 1010 )(0.066) 3.7 1011
pH = pKw - pOH
pH = 14 – 5.2 = 8.8
6
Aqueous
Equilibria
x [OH ] 6.110
pOH log(6.1106 ) 5.2
2005B Q1
Aqueous
Equilibria
Mole ICEnd Table:
Aqueous
Equilibria
(3) Titration of a WB with a SA
SA (H3O+)
MOH ↔ M+ + OHH3O+ + MOH 2H2O + M+
• The pH at the
equivalence
point in these
titrations is < 7.
• Methyl red is the
indicator of
choice.
Aqueous
Equilibria
(3) Titration of a WB with a SA:
Calculation of pH
(1) Acid Base Neutralization
(2) WB Equilibrium problem
B+ H2O ↔ BH+ + OH[ BH ][OH ]
KB
[ B]
-
B + H+ H2O + A
[ BH ]
pOH pKB log
[ B]
Weak base and
strong acid
Use: Mole ICEnd Table
Mole
B
+
(.15M)(.025L)
H+
→
(.10M)(.030L)
A-
Use: [ICE] Table
+ H20
B
II
0.0038 η
0.0030 η
0 η
II
C
- 0.0030 η
- 0.0030 η
+ 0.0030 η
0.0008 η
0 η
0.0030 η
End
↔
BH+
+ H2O
OH-
0.061
0
C
-x
+x
Aqueous
Equilibria +x
0
E
0.061 - x
x
x
2007A Q1
Titration: weak acid with strong base
Aqueous
Equilibria
Use: Mole ICEnd Table
Mole
HA
+
(.40 M)(.025L)
→
A-
II
0.010 η
0.0060 η
0 η
C
- 0.0060 η
- 0.0060 η
+ 0.0060 η
0.0040 η
0 η
End
(e) What’s pH?
OH(.40M)(.015L)
+ H20
0.0060 η
(0.006)
solu
= 0.15 M F
Lso ln (0.015 0.025) L
Aqueous
(0.004 )
solu
Equilibria
For HF: M
= 0.10
M HF
Lso ln
(0.015 0.025) L
For F-: M
0.15 M F
-
0.10 M HF
Use: [ICE] Table
H+
HA
↔
A-
II
0.10
0
0.15
C
-x
+x
+x
E
0.10 - x
x
0.15 + x
Aqueous
Equilibria
Titrations of Polyprotic Acids
Ka3
Ka2
In these
cases there is
an
equivalence
point for each
dissociation.
Ka1
Aqueous
Equilibria
2005B Q1
Titration: weak
acid strong base
Aqueous
Equilibria
2005B Q1
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
2000 QA
Titration: weak
base strong acid
Aqueous
Equilibria
Aqueous
Equilibria
Titration: weak
2001 Q3
acid strong base
Aqueous
Equilibria
Answers 2001 Q3
Aqueous
Equilibria
2002A Q1
Titration: weak
acid strong base
Aqueous
Equilibria
Aqueous
Equilibria
2003A Q1
Titration: weak
base strong acid
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
2006B Q1
Titration: weak
acid strong base
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
Expressing
Concentrations of
Solutions:
Molarity (& Normality*)
* For MDC students only!
Aqueous
Equilibria
Molarity (M)
=
moles of solute
Liters of solution
xA HN + xB MOH MN + HOH
mol A
xA
=
mol B
xB
(mol/L)A x LA
xA
=
(mol/LB) x LB
xB
MA x VA = MB x VB
xA
Where
xA or B
xB
= coefficients for the acid (A) and the base (B)
balanced neutralization equations
Aqueous
Equilibria
from the
mol A
xA
(mol/L)A x LA
xA
MA x VA
molesA
=
=
=
=
mol B
xB
(mol/LB) x LB
xB
xA M
B
xB
xA
x VB
MB x VB
xB
Aqueous
Equilibria
xA HN + xB MOH MN + HOH
For titrations:
Since
1 mole
mole sA # g soluteA
g - MM A
xA
# g solute
g - MM
=
xB
MB x VB
Aqueous
Equilibria
Lesson for MDC students only:
Molarity (M) vs. Normality (N)
M=
mol of solute
L of solution
1 mole
mole s # g Solute
g - MM
equiv of solute
N=
L of solution
1 mole
e quivale nt
s # g Solute
g - EW
g - EW
M=N
When n = 1
That is when using HCl, KHP NaOH
But not when using H2SO4, Ca(OH)2
g - MM
n
Where:
n
A/B
= # H+ or #OH-
n
Aqueous
=
#e
involved
in
balanced
Redox
Equilibria
redox equation.
Molarity (M) vs. Normality (N)
Acid
g-MM (g/)
Molarity (/L) Normality (eq/L)
+ H20 to
g-EW
HCl
36 g
+ 1L = 1M
=
H2SO4
98 g
+ 1L = 1M
=
H3PO4
98 g
+ 1L = 1M
=
Eq(g/gEW)
1N 36/1 36/36
=36
2N 98/2 98/49
=49
3N 98/3 98/33
=32.7 Aqueous
Equilibria
2H3PO4 + 3 Ca(OH)2 6 HOH + Na3PO4
2M H3PO4 3M Ca(OH)2 Using Molarity
1N H3PO4 1N Ca(OH)2
Using Normality
N = nM or M = N
n
Using Normality for titrations:
NA x VA = NB x VB
Aqueous
Equilibria
Titration of a WA with a SB
•
Unlike in the previous
case, the conjugate
base of the acid affects
the pH when it is
formed.
•
The pH at the
equivalence point will
be >7.
Phenolphthalein is
commonly used as an
indicator in these
titrations.
•
Aqueous
Equilibria
Titration: measuring the
+
Equivalence Point (H = OH )
Methyl red
in base
(range R4-6Y)
Phenolphthalein
in base
(range C 8-10 F)
A pH meter or indicators are used to determine when the
solution has reached the equivalence point, at which the
Aqueous
stoichiometric amount of acid equals that of base. The endEquilibria
point of a titration is when indicator changes color.
Titration of a SA with a SB
1. From the start of the titration
the pH goes up slowly. Just
before the equivalence point,
the pH increases rapidly.
2. At the equivalence point,
moles H+ = moles OH-,
and the solution contains
only water and the salt
from the cation of the base
and the anion of the acid.
3. Just after the equivalence
point, the pH increases
rapidly. As more base is
added, the increase in pH
again levels off.
xA HN + xB MOH MN(aq) + HOH
Excess base
H+ = OH-
Excess acid
Aqueous
Equilibria
Solution of unknown
concentration
(M1 x V1 = #mol1)
Titration
Solution of known
concentration
(M2 x V2 = #mol2)
Aqueous
Equilibria
{*TitrationMovie}
Neutralization:
1 mola = 1 molb
xb
(equivalence point) xa
Stoichiometric/Volumetric Calculations
xA HN + xB MOH MN + HOH
ACID
ACID
ACID
xA
xB
BASE
#g
MW
A
xA
BASE
=
MA x VA
xA
=
BASE
MB x VB
xB
=
#g
MW Aqueous
B
xB
Equilibria
2006 (B)
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
2007 (A)
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria
Aqueous
Equilibria