A weak acid, HA
advertisement
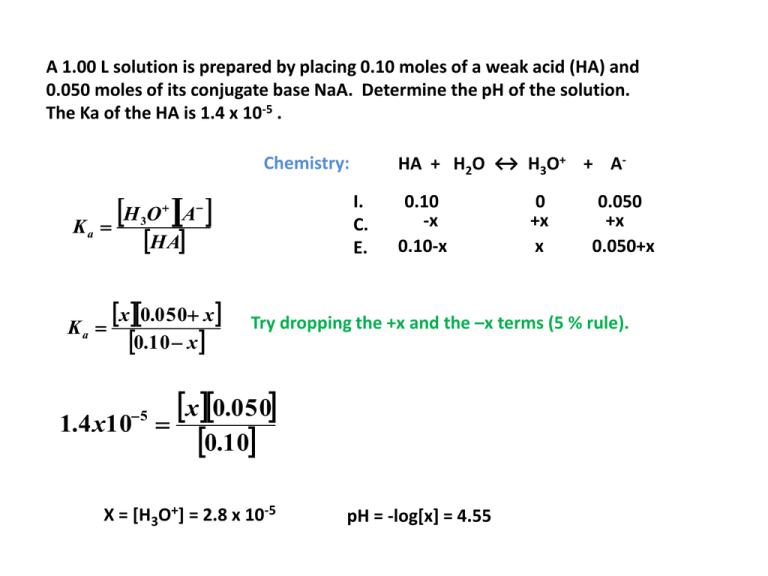
A 1.00 L solution is prepared by placing 0.10 moles of a weak acid (HA) and 0.050 moles of its conjugate base NaA. Determine the pH of the solution. The Ka of the HA is 1.4 x 10-5 . HA + H2O ↔ H3O+ + A- Chemistry: H O A Ka I. C. E. 3 HA Ka x0.050 x 0.10 x 5 1.4 x10 0.10 -x 0.10-x 0 +x x 0.050 +x 0.050+x Try dropping the +x and the –x terms (5 % rule). x 0.050 0.10 X = [H3O+] = 2.8 x 10-5 pH = -log[x] = 4.55 A 1.00 L solution is prepared by placing 0.10 moles of a weak base (B) and 0.050 moles of its conjugate acid BHCl. Determine the pH of the solution. The Kb of the B is 1.4 x 10-5 . B + H2O ↔ OH- + BH+ Chemistry: BH OH Kb I. C. E. B Kb x 0.050 x 0.10 x 5 1.4 x10 0.10 -x 0.10-x 0 +x x 0.050 +x 0.050+x Try dropping the +x and the –x terms (5 % rule). x 0.050 0.10 X = [OH-] = 2.8 x 10-5 pOH = -log[x] = 4.55 and pH = 9.45 A solution is 0.095 M in ascorbic acid and 0.055 M in sodium hydrogen ascorbate. Determine the pH of the solution. Ka1 of the H2C6H6O6 is 6.8 x 10-5, Ka2 = 2.8 x10-12 . Chemistry: H O HC H O Ka 3 6 6 H 2C6 H 6O6 Ka x 0.055 x 0.095 x 6.8 x105 I. C. E. 6 H2C6H6O6 + H2O ↔ H3O+ + HC6H6O60.095 -x 0.095-x 0 +x x 0.055 +x 0.055+x Try dropping the +x and the –x terms (5 % rule). x 0.055 0.095 X = [H3O+] = 1.17 x 10-4 pH = -log[x] = 3.94 A weak acid, HA (Ka = 1.6 x 10-5), is being titrated with a strong base, KOH. Determine the pH of the solution resulting from the mixing of 30.00 mL of 0.10 M HA with 12.00 mL of 0.10 M KOH. First we must determine what is present after the initial reaction between the acid and the base. first reaction: HA(aq) + KOH(aq) → H2O(l) + KA(aq) + XS-HA?? 3 Find starting moles of HA and moles of KOH. Now, Let’s examine the resulting equilibrium. Starting moles HA: 30.00 x10 L 0.10 mole L 3 Starting moles KOH: 3 3.0x10 mol HA 1molOH 3 12 .00 x10 L 0.10 mol KOH 1.2x10 mol OH L 1mol KOH Therefore, we have 1.8 x 10-3 moles of HA left and 1.2 x 10-3 moles of its conjugate base (A-) formed. We can now change these amounts to Molarity and use them in our equilibrium expression. A weak acid, HA (Ka = 1.6 x 10-5), is being titrated with a strong base, KOH. Determine the pH of the solution resulting from the mixing of 30.00 mL of 0.10 M HA with 12.00 mL of 0.10 M KOH. first reaction: HA(aq) + KOH(aq) → H2O(l) + KA(aq) + XS-HA?? 3 Find starting moles of HA and moles of KOH. Now, Let’s examine the resulting equilibrium. Starting moles HA: 30.00 x10 L 0.10 mole L 3 Starting moles KOH: 3 3.0x10 mol HA 1molOH 3 12 .00 x10 L 0.10 mol KOH 1.2x10 mol OH L 1mol KOH Therefore, we have 1.8 x 10-3 moles of HA left and 1.2 x 10-3 moles of its conjugate base (A-) formed. We can now change these amounts to Molarity and use them in our equilibrium expression. HA + H2O ↔ H3O+ + A0 0.0286 I. 0.0429 +x +x C. -x E. 0.0429-x x 0.0286+x A weak acid, HA (Ka = 1.6 x 10-5), is being titrated with a strong base, KOH. Determine the pH of the solution resulting from the mixing of 30.00 mL of 0.10 M HA with 12.00 mL of 0.10 M KOH. Now, Let’s examine the resulting equilibrium. Therefore, we have 1.8 x 10-3 moles of HA left and 1.2 x 10-3 moles of its conjugate base (A-) formed. We can now change these amounts to Molarity and use them in our equilibrium expression. H O A _ 3 Ka HA 5 1.6x10 x0.0286 x 0.0429 x x = [H3O+] = 2.4 x 10-5 pH = -log [2.4 x 10-5] = 4.62 HA + H2O ↔ H3O+ + A0 0.0286 I. 0.0429 +x +x C. -x E. 0.0429-x x 0.0286+x Try dropping the -x and +x terms. If the value of x comes out to less than 5% of 0.0286, dropping the term is justified. A weak acid, HA (Ka = 1.6 x 10-5), is being titrated with a strong base, KOH. Now try: Determine the pH of the solution resulting from the mixing of 30.00 mL of 0.10 M HA with 22.00 mL of 0.10 M KOH. First we must determine what is present after the initial reaction between the acid and the base. first reaction: HA(aq) + KOH(aq) → H2O(l) + KA(aq) + XS-HA?? 3 Find starting moles of HA and moles of KOH. Now, Let’s examine the resulting equilibrium. Starting moles HA: 30.00 x10 L 0.10 mole L 3 Starting moles KOH: 3 3.0x10 mol HA 1molOH 3 22 .00 x10 L 0.10 mol KOH 2.2x10 mol OH L 1mol KOH Therefore, we have 0.8 x 10-3 moles of HA left and 2.2 x 10-3 moles of its conjugate base (A-) formed. We can now change these amounts to Molarity and use them in our equilibrium expression. A weak acid, HA (Ka = 1.6 x 10-5), is being titrated with a strong base, KOH. Determine the pH of the solution resulting from the mixing of 30.00 mL of 0.10 M HA with 12.00 mL of 0.10 M KOH. first reaction: HA(aq) + KOH(aq) → H2O(l) + KA(aq) + XS-HA?? 3 Find starting moles of HA and moles of KOH. Now, Let’s examine the resulting equilibrium. Starting moles HA: 30.00 x10 L 0.10 mole L 3 Starting moles KOH: 3 3.0x10 mol HA 1molOH 3 22 .00 x10 L 0.10 mol KOH 2.2x10 mol OH L 1mol KOH Therefore, we have 0.8 x 10-3 moles of HA left and 2.2 x 10-3 moles of its conjugate base (A-) formed. We can now change these amounts to Molarity and use them in our equilibrium expression. HA + H2O ↔ H3O+ + A0 0.0423 I. 0.0154 +x +x C. -x E. 0.0154-x x 0.0423+x A weak acid, HA (Ka = 1.6 x 10-5), is being titrated with a strong base, KOH. Determine the pH of the solution resulting from the mixing of 30.00 mL of 0.10 M HA with 12.00 mL of 0.10 M KOH. Now, Let’s examine the resulting equilibrium. Therefore, we have 1.8 x 10-3 moles of HA left and 1.2 x 10-3 moles of its conjugate base (A-) formed. We can now change these amounts to Molarity and use them in our equilibrium expression. H O A _ 3 Ka HA 5 1.6x10 x0.0423 x 0.0154 x x = [H3O+] = 2.4 x 10-5 pH = -log [5.825 x 10-5] = 5.23 HA + H2O ↔ H3O+ + A0 0.0423 I. 0.0154 +x +x C. -x E. 0.0154-x x 0.0423+x Try dropping the -x and +x terms. If the value of x comes out to less than 5% of 0.0286, dropping the term is justified.







