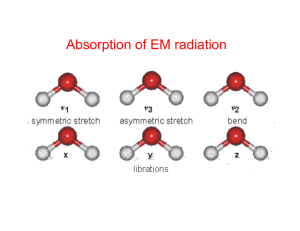
Atomic and molecular vibrations correspond to excited energy levels
advertisement

Absorption of EM radiation Molecular absorption processes ~10-18 J • Electronic transitions • UV and visible wavelengths • Molecular vibrations • Thermal infrared wavelengths Increasing energy • Molecular rotations • Microwave and far-IR wavelengths ~10-23 J • Each of these processes is quantized • Translational kinetic energy of molecules is unquantized Absorption spectra of molecules Hypothetical molecule with three allowed energy levels Note relationship to emission! νij = ΔEij/h (a) allowed transitions (b) positions of the absorption lines in the spectrum of the molecule • Line positions are determined by the energy changes of allowed transitions • Line strengths are determined by the fraction of molecules that are in a particular initial state required for a transition • Multiple degenerate transitions with the same energy may combine Fluorescence • Fluorescent lighting exploits this phenomenon: certain phosphors emit visible light when bombarded with UV light. Much more efficient than incandescent lighting. • Also whitening agents in detergents... Interaction of radiation with matter Wavelength • If there are no available quantized energy levels matching the quantum energy of the incident radiation, then the material will be transparent to that radiation X-ray interactions • Quantum energies of x-ray photons are too high to be absorbed by electronic transitions in most atoms - only possible result is complete removal of an electron from an atom • Hence all x-rays are ionizing radiation • If all the x-ray energy is given to an electron, it is called photoionization • If part of the energy is given to an electron and the remainder to a lower energy photon, it is called Compton scattering Ultraviolet interactions • Near UV radiation (just shorter than visible wavelengths) is absorbed very strongly in the surface layer of the skin by electron transitions • At higher energies, ionization energies for many molecules are reached and the more dangerous photoionization processes occur • Sunburn is primarily an effect of UV radiation, and ionization produces the risk of skin cancer UV SO2 and O3 absorption spectra Visible light interactions • Visible light is also absorbed by electron transitions • Higher energies at blue wavelengths relative to red wavelengths: hence red light is less strongly absorbed than blue light • Absorption of visible light causes heating, but not ionization • Car windshields transmit visible light but absorb higher UV frequencies Infrared (IR) interactions • Quantum energy of IR photons (0.001-1.7 eV) matches the ranges of energies separating quantum states of molecular vibrations • Vibrations arise as molecular bonds are not rigid but behave like springs Microwave interactions • Quantum energy of microwave photons (0.00001-0.001 eV) matches the ranges of energies separating quantum states of molecular rotations and torsion • Note that rotational motion of molecules is quantized, like electronic and vibrational transitions associated absorption/emission lines • Absorption of microwave radiation causes heating due to increased molecular rotational activity • Most matter transparent to µ-waves, microwave ovens use high intensity µ-waves to heat material Molecular dipole moments For a molecule to absorb IR radiation it must undergo a net change in dipole moment as a result of vibrational or rotational motion. The electric dipole moment for a pair of opposite charges of magnitude q is the magnitude of the charge times the distance between them, with direction towards the positive charge. The total charge on a molecule is zero, but the nature of chemical bonds is such that positive and negative charges do not completely overlap in most molecules. Such molecules are said to be polar because they possess a permanent electric dipole moment. Water is a good example of a polar molecule: Molecules with mirror symmetry like oxygen, nitrogen and carbon dioxide have no permanent dipole moments. Key atmospheric constituents • Diatomic, homonuclear molecules (e.g., N2, O2) have no permanent electric dipole moment (also CO2) • Molecular N2, the most abundant atmospheric constituent, has no rotational absorption spectrum • Oxygen (O2) has rotational absorption bands at 60 and 118 GHz • Linear and spherical top molecules have the fewest distinct modes of rotation, and hence the simplest absorption spectra • Asymmetric top molecules have the richest set of possible transitions, and the most complex spectra No • Note lack of permanent electric dipole moment in CO2 and CH4 Vibration modes of simple molecules Fundamental or normal modes 1 2 3 Symmetric stretch Bend (Scissoring) Asymmetric stretch A normal mode is IR-active if the dipole moment changes during mode motion. Overtones, combinations and differences of fundamental vibrations are also possible (e.g., 2v1, v1+v3 etc.) A non-linear molecule of N atoms has 3N-6 normal modes of vibration; a linear molecule has 3N-5. Absorption frequency for a diatomic molecule 1 2c k(m1 m2 )Av m1m2 m1, m2 = atomic mass of vibrating atoms c = speed of light [3×108 m s-1] V = wavenumber [cm-1] Av = Avogadro’s number [6.023×1023 atoms mole-1] k = force constant (bond strength) [dynes cm-1] For a single bond, k = 5×105 dynes cm-1 For a double bond, k = 10×105 dynes cm-1 For a triple bond, k = 15×105 dynes cm-1 Infrared (IR) interactions Vibrational transitions are associated with larger energies than ‘pure’ rotational transitions. Vibrations can be subdivided into two classes, depending on whether the bond length or angle is changing: • Stretching (symmetric and asymmetric) • Bending (scissoring, rocking, wagging and twisting) Stretching frequencies are higher than corresponding bending frequencies (it is easier to bend a bond than to stretch or compress it) Bonds to hydrogen have higher stretching frequencies than those to heavier atoms. Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds Infrared (IR) interactions Region Wavelength [µm] Energy [meV] Wavenumber Type of [cm-1] excitation Far IR 50 - 1000 1.2 - 25 10 – 200 Lattice vibrations, Molecular rotations Mid IR 2.5 - 50 25 - 496 200 - 4000 Molecular vibrations Near IR 1 - 2.5 496 - 1240 4000 - 10000 Overtones Absorption spectra of molecules V = Vibrational quantum number J = Rotational quantum number • Electronic, vibrational and rotational energy levels are superimposed • The absorption spectrum of a molecule is determined by all allowed transitions between pairs of energy levels, and whether the molecule exhibits a sufficiently strong electric or magnetic dipole moment (permanent or otherwise) to interact with the radiation field Vibrational-rotational transitions P branch (ΔJ = -1) Q branch (ΔJ = 0) (pure vibration) R branch (ΔJ = +1) • Relative positions of transitions in the absorption spectrum of a molecule Hydrogen chloride (HCl) spectrum Q branch (ΔJ = 0) P branch R branch • Vibrational-rotational absorption spectrum of HCl: shows affect of two chlorine isotopes with slightly different mass Transmittance spectrum for ozone (O3) http://www.spectralcalc.com/calc/spectralcalc.php Transmittance spectrum for CO2 http://www.spectralcalc.com/calc/spectralcalc.php Transmittance spectrum for H2O http://www.spectralcalc.com/calc/spectralcalc.php Absorption line shapes • Doppler broadening: random translational motions of individual molecules in any gas leads to Doppler shift of absorption and emission wavelengths (important in upper atmosphere) • Pressure broadening: collisions between molecules randomly disrupt natural transitions between energy states, so that absorption and emission occur at wavelengths that deviate from the natural line position (important in troposphere and lower stratosphere) • Line broadening closes gaps between closely spaced absorption lines, so that the atmosphere becomes opaque over a continuous wavelength range. Pressure broadening • Absorption coefficient of O2 in the microwave band near 60 GHz at two different pressures. Pressure broadening at 1000 mb obliterates the absorption line structure. Sulfur dioxide (SO2) ν1: 1151 cm-1, 8.6 µm ν3: 1361 cm-1, 7.3 µm ν2: 519 cm-1, 19.2 µm Sulfur dioxide (SO2) ν1+ν3: 2500 cm-1, 4 µm Water vapor (H2O) • Most important IR absorber • Asymmetric top → Nonlinear, triatomic molecule has complex line structure, no simple pattern • 3 vibrational fundamental modes o H o H symmetric stretch v1 = 2.74 μm bend v2 = 6.25 μm asymmetric stretch v3 = 2.66 μm • Higher order vibrational transitions (Δv >1) give weak absorption bands at shorter wavelengths in the shortwave bands • 2H isotope (0.03% in atmosphere) and 18O (0.2%) adds new (weak) lines to vibrational spectrum • 3 rotational modes (J1, J2, J3) • Overtones and combinations of rotational and vibrational transitions lead to several more weak absorption bands in the NIR Transmission spectrum of H2O Explain the peaks in ni…. Carbon dioxide (CO2) • Linear → no permanent dipole moment, no pure rotational spectrum • Fundamental modes: o c o symmetric stretch asymmetric stretch v1 = 7.5 μm => IR inactive v = 4.3 μm 3 • • • • bend v2 = 15 μm bend v2 The v3 vibration is a parallel band (dipole moment oscillates parallel to symmetric axis), transition ΔJ = 0 is forbidden, no Q branch, greater total intensity than v2 fundamental The v2 vibration is perpendicular band, has P, Q, and R branch The v3 fundamental is the strongest vibrational band, but the v2 fundamental is most effective due to “matching” of vibrational frequencies with terrestrial Planck emission function 13C isotope (1% of C in atmosphere) and 17/18O isotope (0.2%) cause a weak splitting of rotational and vibrational lines in the CO2 spectrum IR Absorption Spectrum of CO2 v3 v2 Which is the most potent greenhouse gas? Ozone (O3) • Ozone is primarily present in the stratosphere except anthropogenic ozone pollution which exists in the troposphere • Asymmetric top → similar absorption spectrum to H2O due to similar configuration (nonlinear, triatomic) • Strong rotational spectrum of random spaced lines • Fundamental vibrational modes o o o symmetric stretch v1 = 9.01 μm o bend v2 = 14.3 μm asymmetric stretch v3 = 9.6 μm – 14.3 μm band masked by CO2 15 μm band – Strong v3 band and moderately strong v1 band are close in frequency, often seen as one band at 9.6 μm – 9.6 μm band sits in middle of 8-12 μm H2O window and near peak of terrestrial Planck function – Strong 4.7 μm band but near edge of Planck functions IR Absorption Spectrum of O3 v1/v3 v2 Methane (CH4) • Spherical top • 5 atoms, 3(5) – 6 = 9 fundamental modes of vibration • Due to symmetry of molecule, 5 modes are degenerate, only v3 and v4 fundamentals are IR active • No permanent dipole moment => No pure rotational spectrum • Fundamental modes H C C H C C H H v1 v2 v3 = 3.3 µm v4 = 7.7 µm IR Absorption Spectrum of CH4 v3 v4 • 7.6 µm band in otherwise largely transparent part of atmosphere • Methane concentrations also directly/indirectly affected by human activities Nitrous oxide (N2O) • Linear, asymmetric molecule (has permanent dipole moment) • Has rotational spectrum and 3 fundamentals • Absorption band at 7.8 μm broadens and strengthens methane’s 7.6 μm band. • 4.5 μm band less significant as it is at the edge of the Planck function. • Fundamental modes: O N N symmetric stretch v1 = 7.8 μm asymmetric stretch v3 = 4.5 μm bend v2 bend v2 = 17.0 μm IR Absorption Spectrum of N2O v3=4.5 µm v1=7.8 µm v2=17 µm Mineral and rock reflectance spectra • Electronic transitions in solids; Fe2+ (iron) particularly important in remote sensing – minerals contain Fe2+ ions • Fundamental vibrational modes of H2O: 2.74 µm, 6.25µm, 2.66 µm • In rock spectra, whenever water is present we see 2 absorption bands in near-IR spectra – one near 1.45 µm (2ν3 overtone) and one near 1.9 µm (v2+v3 combination). Sharpness of bands relates to sites in crystal structure occupied by the water molecules. • Note that penetration depth into natural surfaces is usually restricted to the upper few microns. Consequences? Geological mapping/prospecting Escondida Mine, Atacama Desert, Chile ASTER visible ASTER short-wave IR (SWIR) Why are most plants green and then red or yellow in the fall? • Chlorophyll absorbs in the red and blue, and hence reflects in the green. • Its absorption spectrum is due to electronic transitions In the fall, trees produce carotenoids, which reflect yellow, and anthocyanins, which reflect orange and red.