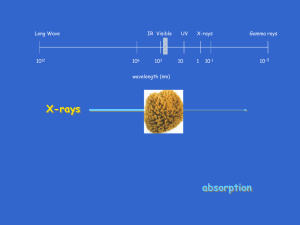
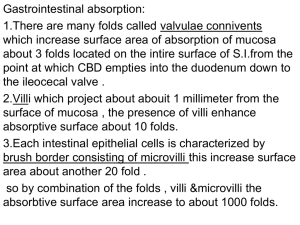
Absorption analysis at different pH levels
advertisement
Glucose absorption analysis at different pH levels Erich Awender (Introduction) Matt Lopez (Method) Mitch Powers (Results) Hasani Morrow (Discussion) Introduction Goal: • The goal of our experiment is to demonstrate how different pH levels will affect the break down of glycogen. Hypothesis: • The absorption of glucose at 540 nm will be affected by different levels of pH. Predictions: • Higher absorption at a lower pH • Lower absorption at a higher pH Background info • A previous study by Zuping Tang, Xiaogu Du, Richard Louie, and Gerald Kost was very similar to ours – Also tested glucose absorption at higher and lower pH levels – Found that with normal glucose levels the difference in pH was not significant – However, increased levels of glucose showed a higher absorption at higher pH and lower absorption at lower pH First off we labeled the tubes so we did not mix them up, then we added the saliva solution to all the tubes. Then we filled three tubes with a high pH, three with a low pH, three with a neutral pH, and one without any added pH since it was the control in the experiment. Afterwards we added the DNSA and buffer at the same time intervals so we could be as accurate as possible. After we got all the test tubes ready we put them on a hot plate and we started to turn up the heat to get them bubbling a bit. We kept them on the hot plate for five minutes then took them out and put them back into the racks into their groups. After we had all the test tubes separated we put small samples of each mixture and put samples of them into cuvettes and lined them up based on their group. After we had all that done we put them into the spectrometer and recorded the absorbance they showed at 540 nm. • Dnsa – When DNSA is reduced it yields 3 amino, 5 nitrosalicylic acid, and deeply colors a compound which absorbs strongly at 540nm • The relationship between change in color for the acids and bases were generally the same color but were a little darker, while the neutral was the darkest one because it absorbed the most glucose while the acidic and basic test tubes absorbed less and were generally the same color. • pH is used as a scale to show how acidic or basic a certain substance is. Less than 7 is acidic, 7 is neutral, and more than 7 is basic. A buffer is a solution that has a very stable pH, and adding an acid or base should not affect it much and adding water should not change the pH of the buffer. Results The Basic and Acidic glycogen are not significantly different with a p value =(0.37) The Basic and Neutral glycogen are significantly different with a p value=(.04) The Acidic and Neutral glycogen are significantly different with a p value =(.03) Let’s Discuss Shall We • Our hypothesis was that the absorption will be affected by different PH’s • We predicted that there will be a higher absorption at a lower PH • A lower absorption at a higher PH • And that the neutral PH would fall in between the two extremes Our Expectations • The different PH’s did affect the absorption of the glucose however not in the way we predicted. • We thought that a higher PH would result in less absorption and that a lower PH would result in more, but the absorption levels for both were very similar. Basic having a higher absorption level by only .001nm • The neutral PH which we thought would be in between the acidic and basic actually had the highest amount of absorption. Similar Studies • According to a similar experiment done by Zuping Tang, MD; Xiaogu Du, MD; Richard F. Louie, BS; Gerald J. Kost, MD, PhD in 2000 the higher PH resulted in higher absorption and the lower resulted in lower absorption. • Compared to our results theirs were significantly different however in the article it said they were using much more sophisticated equipment. • "Archives of Pathology & Laboratory Medicine Online - Error." Archives of Pathology & Laboratory Medicine Online - Error. N.p., n.d. Web. 26 Sept. 2012. <http://www.archivesofpathology.org/doi/pdf/10.1043/00039985(2000)124%3C0577%3AEOPOGM%3E2.0.CO%3B2>. So What Now? • The questions that we had after our experiment were: • Why was the neutral PH so much higher? • Why were the acidic and basic PH’s so similar?