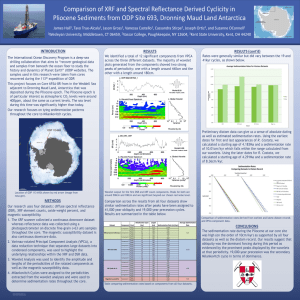
Sedimentation
advertisement

By: Ashley and Christine
Phy 200
Professor Newman
4/13/12
What is it?
Technique used to settle particles in solution against the
barrier using centrifugal acceleration
Two Types of Centrifuges
Analytical
Non Analytical
History
1913 - Dumansky proposed the use of ultracentrifugation to determine
dimensions of particles
1923 - The first centrifuge is constructed by Svedberg and Nichols.
1929 - Lamm deduces a general equation that describes the movement
within the ultracentrifuge field
1940s - Spinco Model E centrifuge becomes commercially available
1950s - Sedimentation becomes a widely used method.
1960s - First scanning photoelectric absorption optical system developed
1980s - Sedimentation loses popularity due to data treatment being slow
and the creation of gel electrophoresis and chromatography
1990s - Newer versions of centrifuge gains popularity again
2000s - It is now recognized as a necessary technique for most laboratories
How Does It Work?
Everything has a sedimentation coefficient
Ratio of measured velocity of the particle to its centrifugal
acceleration
Can be calculated from the forces acting on a particle in the
cell
Sedimentation Coefficient
Usually, determine mass by observing movement of
particles due to known forced
Use Gravity normally
For molecules, force is too small
Avoid this by increasing PE by putting paricles in a cell
rotating at a high speed
Get Sedimentation Coefficient
How does it work?
Rotors must be capable of withstanding large
gravitational stress
Two types of cells: double sector (accounts for
absorbing components in solvent) and
boundary forming (allows for layering of
solvent over the solution)
Optical detection systems: Rayleigh optical
system (displays boundaries in terms of
refractive index as a function of radius),
Schlieren optical system (refractive index
gradient as a function of radius), and
absorption optical system (optical density as a
function of radius)
Data acquisition is computer automated due
to the Beckman Instruments Optima XL
analytical centrifuge
Deriving the Lamm Equation
Describes the transport process in the ultracentrifuge
Fick’s first equation:
Jx = -D[dC/dx]
If all particles in the cell drift in a +x direction at speed, u:
Jx = -D[dC/dx] + uC(x)
u = sω2x
Therefore, Jx = -D[dC/dx] + sω2xC(x)
For ideal infinite cell lacking walls.
Lamm Equation
For real experimental conditions:
Cross-section of a sector cell is proportional to r
Continuity equation:
(dC/dt)r= -(1/r)(drJ/dr)t
Combine ideal equation with continuity equation to obtain:
(dC/dt)r = -(1/r){(d/dr)[ω2r2sC – Dr(dc/dr)t]}t
Describes diffusion with drift in an AUC sector cell under
real experimental conditions.
http://www.nibib.nih.gov/Research/Intramural/lbps/pbr/auc/LammEqSoluti
ons
Lamm Equation: Different
Boundary Conditions
Exact Solutions Exist in 2 limiting cases:
1. “NO DIFFUSION”
Homogeneous macromolecular solution
C2(x,t) = {0
if xm<x<xavg
{C0exp(-2sω2t)
if xavg<x<xb
2. “NO SEDIMENTATION”
Lamm Equation: (dC/dt)r = -D(d2C/dt2)t
Concentration Gradient: (dC/dt)r = -Co(πDt)1/2exp(-x2/4Dt)
Diffusion coefficient determined by measuring the standard
deviation of Gaussian curve
Used for small globular proteins, at low speed, with synthetic
boundary cell.
Technology Enabling Analytical
Analysis
Two computer modeling methods enable simultaneous
determination of sedimentation, diffusion coefficients, and
http://www.aapsj.org/view.asp?art=aa
molecular mass.
psj080368
1. vanHolde-Weischet Method:
Extrapolation to infinite time must eliminate the contribution
of diffusion to the boundary shape.
ULTRASCAN software
2.Stafford Method:
Sedimentation coefficient distribution is computed from the
time derivate of the sedimentation velocity concentration
profile
http://www.ultrascan2.uthscsa.edu/tutorial/basics_5.html
Specific Boundary Conditions
Faxen-type solutions:
Centrifugation cell considered infinite sector
Diffusion is small
Only consider early sedimentation times
Archibald solutions:
S and D considered constant
Fujita-type solutions:
D is constant
S depends on concentration
VelocityEquilibrium
Sedimentation
Sedimentation Sedimentation
Velocity and
Equilibrium
Angular Velocity
Large
(according to
sedimentation
properties)
Small
Analysis
As a function of time
At equilibrium
Measurement
Forming a Boundary
Particle distribution in
cell
Calculated Parameters
Shape, mass composition Mass composition
Sedimentation Velocity
How we measure the results: Determine the Sedimentation
and Diffusion Coefficients from a moving boundary
It takes a While to Run an
Experiment!
Speed (rpm)
Time at each speed (s) Svedbergs
0-6000
15
500
6000
600
4220
9000
600
1330
13000
600
550
18000
600
250
25000
600
125
50000
3600
31
Correcting to Standard Value
Allows for standardization of sedimentation coefficients
Concentration Dependence
Sedimentation coefficients of biological macromolecules
are normally obtained at finite concentration and should
be extrapolated to zero concentration
Determining Macromolecular Mass
First Svedberg equation:
M = sRT/D(1-υavgρo)
Assumptions:
Frictional coefficients affecting diffusion and sedimentation
are identical
Sedimentation Equilibrium
Even if centrifuged for an extended period of time,
macromolecules will not join pellet because of
gravitational and diffusion force equilibrium.
Molecular mass determination is independent of shape.
Shape only affects rate equilibrium is reached, not
distribution.
No changes in concentration with time at equilibrium
Total flux = 0
Binding Constants
Can measure concentration dependence of an effective
average molecular mass.
Can be used to describe different kinds of phenomenon.
Dissociation equilibrium constant can be directly
determined from the equilibrium sedimentation data
C(r) = CA(r)σA + CB(r)σB + CAB(r)σAB
Partial Specific Volume
Needed when determining molecular mass through
sedimentation
Measurement of the density of the particle using its
calculated volume and mass
Very difficult to make precise density measurements
needed
Density Gradient Sedimentation
Velocity Zonal Method
Layered density gradient
Sucrose, glycerol
Particles separate into zones based on sedimentation velocity,
according to sedimentation coefficients
Determined by size, shape, and buoyant density
Estimation of molecular masses
Potential Problem:
Molecular crowding effect due to high sucrose concentration
Density Gradient Sedimentation Equilibrium
Density gradient itself formed by centrifugal field
Used in experiment by Messelson and Stahl
http://www.mun.ca/biology/scarr/Gr10-23.html
Molecular Shape
Sedimentation coefficient
dependent on particle volume
and shape
Molecules having the same
shape, but different molecular
mass form a homologous
series.
http://web.virginia.edu/Heidi/chapter30
/chp30.htm