Comparison of the Aggregation Behavior of TiO 2 Nanoparticles

1
2
3
4
5
6
Comparison of the Aggregation Behavior of TiO
2
Nanoparticles
7
Exposed to Fulvic Acid and Bacillus subtilis Exudates
8
9
Supplementary Material
10
11
12
13
14
15
T HOMAS A.
D USTER
1*
AND J EREMY B.
F EIN
1
1
Department of Civil and Environmental Engineering and Earth Science,
University of Notre Dame, Notre Dame, IN 46556, USA
[*Present Correspondence: thomas.duster@nist.gov / 303.497.3486 (Ph) / 303.497.6682 (Fax)]
S1
16 S1. M
ATERIALS AND
S
TOCK
S
OLUTION
P
REPARATION
17 A 10 g L -1 stock of commercially-obtained nano-TiO
2
(anatase; Sigma-Aldrich, USA)
18
19 was created by suspending 1 gram of nanoparticles in 100 mL of ultrapure water (R>18 MΩ cm,
TOC < 2μg C L -1
). The details and physiochemical characteristics of the nano-TiO
2
utilized in
20 this study are provided in Table S1, as reported by the manufacturer or determined by previous
21 studies using identical particles (Simon-Deckers et al. 2009; Bhardwaj et al. 2010). Upon
22 suspension of the particles, we observed relatively rapid sedimentation, strongly suggesting a
23 high extent of aggregation in the original suspension. Prior to an individual experimental run,
24 the master stock suspension was thoroughly mixed via end-over-end rotation, and a 250 mg L
-1
25 secondary stock suspension was made by removing an aliquot of the master stock suspension and
26 diluting with ultrapure water. This secondary suspension was subjected to ultrasonication in a
27 water bath for at least one hour, and remained there during subsequent distribution to
28 experimental systems.
29 A 200 mg L
-1
fulvic acid stock solution was created by dissolving into ultrapure water a
30 freeze-dried solid Suwannee River fulvic acid (SRFA) standard (Product no. 1S101F) obtained
31 from the International Humic Substances Society. Bacterial exudate preparation followed the
32 procedure outlined by Seders and Fein (2011). Cultures of Bacillus subtilis , an aerobic, Gram-
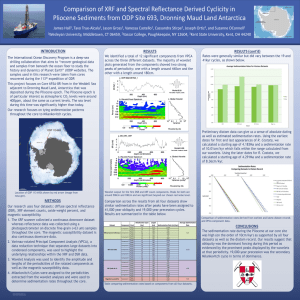
33
34 positive soil bacterium, were initially grown in approximately 3 mL of aerobic trypticase soy broth (TSB; 30 g L
-1 ) spiked with 0.5% yeast extract (YE), and shaken at 100 rpm in a 32° C
35 incubator. After 24 hours, these starts were transferred to 2 L of fresh media comprised of the
36 same TSB/YE mixture and placed back in the incubator with the same settings. After another 24
37 hours, the solution was transferred to 250 mL polypropylene bottles, and cells were harvested via
38 centrifugation at 8000 × g for 5 minutes. Pellets were then resuspended in 0.1 M NaNO
3
via
S2
39 short (<5 second) bursts on a vortex, transferred to 30 mL polypropylene tubes, and again
40 pelleted via centrifugation at 9000 × g for 5 minutes. Cells were resuspended and washed 5
41 times in this manner, and excess electrolyte was then removed after the washing procedure by
42 two subsequent centrifugation runs at 8000 × g for 30 minutes. Bacterial wet masses were
43 obtained, and cells were resuspended in 0.1 M NaNO
3
at a concentration of 40 g (wet bacterial
44 mass) L
-1
. To obtain the necessary quantities of bacteria, approximately 10 L of a 24-hour grown
45 bacterial culture was initially required.
46
47
Exudate was then extracted via gentle end-over-end inversion for 2.5 hours, followed by centrifugation at 8000 × g for 5 minutes to remove bacterial cells. Contrary to the procedures
48 followed by Seders and Fein (2011), pH was not adjusted during the extraction period, and was
49 measured at ~6.8 following rotation. While there was no explicit attempt to lyse cells during this
50 procedure, it should be noted that complex organics extracted in this manner could conceivably
51 include cellular components similar to lysate, as disturbances during the extraction procedure
52 could potentially breach bacterial cell walls. These extractions are not the same as non-aqueous
53 extracellular polymeric substances (EPS) observed during biofilm studies. After extraction of
54 bacterial exudate (and after preparation of SRFA, as well), resulting solutions were filtered
55
56 through 0.2 μm polyethersulfone (PES) filters into pre-sterilized media bottles, and stored in the dark at 4° C for up to 7 days.
57 SRFA and bacterial exudate solutions were analyzed for dissolved organic carbon (DOC;
58 Shimadzu TOC-V), and these values were used to normalize NOM additions in experimental
59 treatments. We expect that proton-active functional groups are likely the primary agents through
60 which organic molecules interact with nano-TiO
2
surfaces. Hence, for reference, Table S2
S3
61 provides the total proton-active site concentrations and acidity constants for B. subtillis exudate
62 and SRFA as reported by Seders and Fein (2011) and Ritchie and Perdue (2003), respectively.
63
64 S2. D
ETAILS
R
EGARDING
D
YNAMIC
L
IGHT
S
CATTERING
M
EASUREMENTS
65 Throughout our experimental range, we regularly observed very large aggregates, with a
66 mean aggregate diameter for the 151 DLS measurements conducted during this study exceeding
67 1500 nm. In combination with the very high density of nano-TiO
2
(~3.9 mg mL -1 ; Table S1), the
68 presence of large aggregates in the DLS samples resulted in rapid sedimentation and non-
69 Brownian characteristics in many of these suspensions. While the DLS data ultimately exhibited
70 a strong relationship to the sedimentation rate parameter (R
2
~0.86; Figure S1), it also suffered
71 from poor analytical precision (Figure S2).
72 The relative dominance between sedimentation and Brownian motion, respectively, in
73 suspensions of spherical particles is illustrated by the Peclet number (P e
):
74
75 P 𝑒
=
4π ∆ρ g a 4
3 K
𝐵
𝑇
76
77 where
∆ρ
is the difference between the densities of particles and the surrounding fluid (kg m
-3
),
78 g is gravitational acceleration (m s
-2
), a is the particle radius (m), K
B
is the Boltzman constant (J
79 K -1 ), and T is the temperature of the solution (K). Aggregates are certainly not spherical
80 particles, and this factor increases drag and slows the sedimentation rate (e.g., similar to
81 complications in clay particle size analysis; (Eshel et al. 2004)), which suggests that a larger P e
is
82 required before non-Brownian properties results in sedimentation. However, large aggregates
83 are also distinguished from spherical particles by their permeability, which is influenced by the
S4
84 packing density of individual particles. Permeability of an aggregate increases the rate at which
85 the aggregate will sediment, thereby increasing the sedimentation term in the equation above
86 (Kim and Yuan 2005; Li and Logan 2001). The relative influence of these two factors in using a
87 Peclet number determination for approximate characterization of sedimentation characteristics of
88 nano-TiO
2
aggregates is unknown.
89 Despite the caveats above, the Peclet number is an effective index for approximating the
90 relative influence of sedimentation and Brownian motion in our systems subjected to DLS
91 analysis. The Peclet numbers for spherical particles with a density identical to the nano-TiO
2
92 used in the present study (~3.9 g mL
-1
; Table S1) is illustrated in Figure S3. Ramaswamy (2001)
93 posits that P e
> 100 approximately distinguishes Brownian from non-Brownian suspensions, but
94 suggests that substantial non-equilibrium contributions to diffusion may be apparent in
95 suspensions at P e
~ 1, or above an aggregate diameter of ~860 nm in our systems. Given that
96 DLS measurements are based on the diffusion of particles in Brownian motion, and more than
97 half of the aggregates measured on the DLS in our systems exceeded a diameter of ~860 nm, we
98 decided that the extensive use of DLS values in our study would not be merited.
99 Figure S2 indicates further evidence of significant complication in DLS measurements.
100 Relative standard deviation (%RSD) values (defined as 100 times the standard deviation of
101 triplicate sample runs divided by their mean) suggest that analytical precision varied widely.
102 However, there does not appear to be a significant trend in these data with respect to aggregate
103
104 diameter, even for aggregates with diameters beyond the analytical maximum of the instrument
(3 μm). To illustrate, the diameter values of these aggregates remain on Figure S2.
105 As a result of these collective complications, DLS measurements are reported primarily
106 for the purposes of general calibration of sedimentation rate data (see Figure S1), as well as to
S5
107 qualitative compare our findings to other literature. We recognize that DLS-based measurements
108 of nano-TiO
2
effective diameters consider all aggregates in suspension and describe their
109 distribution with a mean and variance (i.e., polydispersity index), while the sedimentation rate
110 effectively measures only the fraction of aggregates in these systems that exceed a size large
111 enough to sediment beyond the measurement point within 120 minutes. Therefore, the
112 sedimentation rate metric also does not provide a complete indication of size distribution and
113 measures a slightly different property of sedimenting systems, relative to the DLS measurements.
114 Still, as previously noted, our experimental treatments often resulted in very large nano-TiO
2
115 aggregates, which we did not want to overlook using a selective sampling approach that settled
116 large aggregates prior to DLS-based analysis. Hence, for the purposes of our study, we propose
117 that the sedimentation rate metric could be utilized with fewer restrictions, relative to the DLS
118 measurements, throughout the range of observed aggregation behaviors, and we preferred its use
119 as a proxy for aggregate size distribution.
S6
Table S1.
Physiochemical properties reported for the TiO
2
nanoparticles utilized in this study.
Supplier Product No.
Morphology
Sigma 637254
Sigma-Aldrich 637254
Sigma-Aldrich 637254
1
Trace metal basis
2
At 25 ° C
3
Reference does not identify a method
4 pH 5.5 in ultrapure water
NR - Not reported by reference spherical
NR
NR
Purity
1
NR
NR
99.7%
Density
2 Size SSA ζ 4
(g mL
-1
) (TEM) (BET) (g m
-2
) (mV )
NR 17 nm 12 nm 122 12
NR
3.9
25 ± 2 nm
3
<25 nm
3
117.2 ± 1
200-220
NR
NR pH
PZC
5.6
5.4
NR
Crystalline
Structure Citation
Anatase Simon-Decker et al. 2009
Anatase
Anatase
Bhardwaj et al. 2010
Manufacturer
S6
Table S2.
Functional group site concentrations and acidity constants for each NOM matrix utilized in this study.
Organic Matrix
SRFA
B. subtilis exudate
Total Site
Concentration
14.4 meq gC
~12 meq gC
NR - Not reported by reference
-1
-1 pKa
1
3.8
3.98
pKa
2
NR
6.57
pKa
3
9.52
9.22
Citation
Richie & Perdue 2003
Seders & Fein 2011
S7
Figure S1. Relationship between the mean effective hydrodynamic diameters of nano-TiO
2
aggregates from duplicate experimental batches determined via dynamic light scattering and the corresponding mean sedimentation rates (absorbance lost at 508 nm / min) from the initial 120 minutes of analysis for duplicate experimental batches. The line indicates a linear regression through these points, with associated linear correlation coefficient.
S8
Figure S2.
Analytical precision (as %RSD) for each of the triplicate DLS measurements of nano-TiO
2
aggregates in the present study.
S9
Figure S3.
Peclet number values for spherical particles with various diameters and a density of 3.9 g mL
-1
at T =298K. Note the logarithmic scale on the y-axis.
S10
High IS Treatments Low IS Treatments
1.00
0.80
0.60
0.40
0.20
0.00
pH=3.0 / IS=0.10 / [C]=0.0 / m=2.1
pH=2.9 / IS=0.10 / [C]=0.0 / m=1.9
pH=2.9 / IS=0.10 / [C]=0.0 / m=1.9
pH=3.0 / IS=0.10 / [C]=0.0 / m=2.1
1.00
0.80
0.60
0.40
0.20
0.00
pH=3.0 / IS=0.01 / [C]=0.0 / m=0.2
pH=2.9 / IS=0.01 / [C]=0.0 / m=0.4
pH=2.9 / IS=0.01 / [C]=0.0 / m=0.3
pH=3.0 / IS=0.01 / [C]=0.0 / m=0.4
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
1.00
0.80
0.60
0.40
pH=3.0 / IS=0.10 / [C]=0.1 / m=2.5
pH=0.0 / IS=0.10 / [C]=0.1 / m=2.7
pH=3.0 / IS=0.10 / [C]=0.1 / m=2.4
pH=3.0 / IS=0.10 / [C]=0.1 / m=2.9
0.20
0.00
pH=3.0 / IS=0.10 / [C]=0.9 / m=3.2
pH=3.0 / IS=0.10 / [C]=0.9 / m=3.2
pH=3.0 / IS=0.10 / [C]=1.0 / m=4.3
pH=3.0 / IS=0.10 / [C]=1.0 / m=4.5
1.00
0.80
0.60
0.40
0.20
0.00
pH=2.9 / IS=0.10 / [C]=4.7 / m=3.0
pH=2.9 / IS=0.10 / [C]=4.7 / m=2.6
pH=2.9 / IS=0.10 / [C]=4.7 / m=4.9
pH=3.0 / IS=0.10 / [C]=4.9 / m=5.5
1.00
0.80
0.60
pH=2.9 / IS=0.01 / [C]=0.1 / m=0.2
pH=2.9 / IS=0.01 / [C]=0.1 / m=0.3
pH=3.0 / IS=0.01 / [C]=0.1 / m=0.6
pH=3.0 / IS=0.01 / [C]=0.1 / m=1.0
pH=2.9 / IS=0.01 / [C]=0.9 / m=3.0
pH=3.0 / IS=0.01 / [C]=0.9 / m=3.1
pH=2.9 / IS=0.01 / [C]=1.0 / m=4.5
pH=3.0 / IS=0.01 / [C]=1.0 / m=4.7
pH=2.9 / IS=0.01 / [C]=4.7 / m=1.9
pH=2.9 / IS=0.01 / [C]=4.7 / m=2.6
pH=2.9 / IS=0.01 / [C]=4.7 / m=4.7
pH=3.0 / IS=0.01 / [C]=4.9 / m=5.7
1.00
0.80
0.60
0.40
0.20
0.00
0.40
0.20
0.00
pH=3.0 / IS=0.10 / [C]=9.3 / m=2.7
pH=2.9 / IS=0.10 / [C]=9.3 / m=2.4
pH=2.9 / IS=0.10 / [C]=9.9 / m=5.3
pH=3.0 / IS=0.10 / [C]=9.6 / m=4.8
1.00
0.80
0.60
0.40
0.20
0.40
0.20
pH=3.0 / IS=0.01 / [C]=9.3 / m=0.1
pH=2.9 / IS=0.01 / [C]=9.4 / m=0.3
pH=2.9 / IS=0.01 / [C]=10.0 / m=0.3
pH=3.0 / IS=0.01 / [C]=9.8 / m=0.4
0.00
0.00
60 120 180
Sedimentation Time (min)
240 300 60 120 180
Sedimentation Time (min)
240 300
Figure S4.
Sedimentation plots for nano-TiO
2
aggregates at pH 3 in high (0.1 M; left) and low
(0.01 M; right) IS treatments. NOM concentrations increase from the top panels to the bottom panels within a column. In individual panels, filled symbols indicate the results from duplicate bacterial exudate treatments, while open symbols are for duplicate SRFA treatments. The legends indicate the final pH after 24 hours of exposure, IS (in M) and NOM (mg DOC L
-1
) concentrations, and the sedimentation rate (m; min
-1
) during the initial 120 minutes of sedimentation. Two replicates for each treatment are distinguished. DNM=did not measure.
S11
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
High IS Treatments pH=4.0 / IS=0.10 / [C]=0.0 / m=2.5
pH=4.0 / IS=0.10 / [C]=0.0 / m=2.3
pH=4.1 / IS=0.10 / [C]=0.0 / m=2.3
pH=3.8 / IS=0.10 / [C]=0.0 / m=3.0
1.00
0.80
0.60
0.40
0.20
0.00
pH=4.0 / IS=0.10 / [C]=0.1 / m=2.7
pH=4.0 / IS=0.10 / [C]=0.1 / m=2.7
pH=4.1 / IS=0.10 / [C]=0.1 / m=2.6
pH=4.0 / IS=0.10 / [C]=0.1 / m=3.6
1.00
0.80
0.60
0.40
0.20
0.00
pH=4.0 / IS=0.10 / [C]=0.9 / m=3.2
pH=4.0 / IS=0.10 / [C]=0.9 / m=3.0
pH=4.1 / IS=0.10 / [C]=1.0 / m=4.2
pH=3.9 / IS=0.10 / [C]=1.0 / m=4.6
1.00
0.80
0.60
0.40
0.20
0.00
pH=4.0 / IS=0.10 / [C]=4.6 / m=2.5
pH=4.0 / IS=0.10 / [C]=4.7 / m=2.6
pH=4.0 / IS=0.10 / [C]=5.1 / m=5.3
pH=3.7 / IS=0.10 / [C]=4.8 / m=5.4
1.00
0.80
0.60
Low IS Treatments pH=3.9 / IS=0.01 / [C]=0.0 / m=0.8
pH=4.0 / IS=0.02 / [C]=0.0 / m=0.3
pH=4.0 / IS=0.01 / [C]=0.0 / m=0.3
pH=3.9 / IS=0.01 / [C]=0.0 / m=0.9
pH=3.9 / IS=0.01 / [C]=0.1 / m=1.6
pH=3.9 / IS=0.01 / [C]=0.1 / m=1.2
pH=4.0 / IS=0.01 / [C]=0.1 / m=1.6
pH=3.9 / IS=0.01 / [C]=0.1 / m=3.1
pH=4.0 / IS=0.01 / [C]=0.9 / m=3.1
pH=4.0 / IS=0.01 / [C]=1.0 / m=3.3
pH=4.0 / IS=0.01 / [C]=1.0 / m=4.1
pH=4.0 / IS=0.01 / [C]=1.0 / m=4.5
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
pH=3.9 / IS=0.10 / [C]=9.4 / m=1.5
pH=4.0 / IS=0.10 / [C]=9.4 / m=2.3
pH=4.0 / IS=0.10 / [C]=10.0 / m=4.4
pH=3.9 / IS=0.10 / [C]=9.8 / m=4.5
1.00
0.80
0.60
pH=3.9 / IS=0.01 / [C]=4.7 / m=0.3
pH=4.0 / IS=0.01 / [C]=4.8 / m=0.3
pH=4.0 / IS=0.01 / [C]=5.1 / m=0.3
pH=4.0 / IS=0.01 / [C]=4.9 / m=0.3
0.40
0.20
0.40
0.20
pH=3.9 / IS=0.01 / [C]=9.3 / m=0.3
pH=4.0 / IS=0.01 / [C]=9.4 / m=0.3
pH=4.0 / IS=0.01 / [C]=9.9 / m=0.2
pH=4.0 / IS=0.01 / [C]=9.7 / m=0.3
0.00
0.00
60 120 180
Sedimentation Time (min)
240 300 60 120 180
Sedimentation Time (min)
240 300
Figure S5.
Sedimentation plots for nano-TiO
2
aggregates at pH 4 in high (0.1 M; left) and low
(0.01 M; right) IS treatments. NOM concentrations increase from the top panels to the bottom panels within a column. In individual panels, filled symbols indicate the results from duplicate bacterial exudate treatments, while open symbols are for duplicate SRFA treatments. The legends indicate the final pH after 24 hours of exposure, IS (in M) and NOM (mg DOC L
-1
) concentrations, and the sedimentation rate (m; min
-1
) during the initial 120 minutes of sedimentation. Two replicates for each treatment are distinguished.
S12
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
High IS Treatments pH=5.9 / IS=0.10 / [C]=0.0 / m=3.3
pH=6.1 / IS=0.11 / [C]=0.0 / m=3.1
pH=5.9 / IS=0.11 / [C]=0.0 / m=3.3
pH=5.9 / IS=0.11 / [C]=0.0 / m=3.6
1.00
0.80
0.60
0.40
0.20
0.00
pH=6.0 / IS=0.10 / [C]=0.1 / m=3.1
pH=6.1 / IS=0.11 / [C]=0.1 / m=3.0
pH=5.9 / IS=0.11 / [C]=0.1 / m=3.9
pH=5.9 / IS=0.11 / [C]=0.1 / m=3.8
1.00
0.80
0.60
0.40
0.20
0.00
pH=6.0 / IS=0.10 / [C]=0.9 / m=2.7
pH=6.1 / IS=0.11 / [C]=0.9 / m=2.5
pH=6.0 / IS=0.11 / [C]=0.9 / m=6.5
pH=5.9 / IS=0.11 / [C]=1.0 / m=4.6
1.00
0.80
0.60
0.40
0.20
0.00
pH=6.0 / IS=0.10 / [C]=4.6 / m=2.5
pH=6.1 / IS=0.11 / [C]=4.7 / m=2.2
pH=6.0 / IS=0.11 / [C]=4.8 / m=1.2
pH=5.9 / IS=0.11 / [C]=4.8 / m=3.3
1.00
0.80
0.60
0.40
0.20
0.00
Low IS Treatments pH=5.9 / IS=0.02 / [C]=0.0 / m=3.1
pH=6.1 / IS=0.02 / [C]=0.0 / m=3.2
pH=5.9 / IS=0.02 / [C]=0.0 / m=3.6
pH=5.9 / IS=0.02 / [C]=0.0 / m=3.7
pH=6.0 / IS=0.01 / [C]=0.9 / m=0.2
pH=6.1 / IS=0.02 / [C]=0.9 / m=2.5
pH=6.0 / IS=0.02 / [C]=1.0 / m=1.8
pH=5.9 / IS=0.02 / [C]=0.9 / m=3.3
pH=6.1 / IS=0.01 / [C]=4.7 / m=0.2
pH=6.1 / IS=0.02 / [C]=4.7 / m=0.3
pH=6.0 / IS=0.02 / [C]=4.9 / m=0.8
pH=5.9 / IS=0.02 / [C]=4.9 / m=0.7
pH=6.0 / IS=0.02 / [C]=0.1 / m=2.7
pH=6.1 / IS=0.02 / [C]=0.1 / m=3.0
pH=6.0 / IS=0.02 / [C]=0.1 / m=3.9
pH=5.9 / IS=0.02 / [C]=0.1 / m=4.0
1.00
0.80
0.60
1.00
0.80
0.60
0.40
0.20
pH=5.9 / IS=0.11 / [C]=10.3 / m=0.8
pH=5.9 / IS=0.11 / [C]=9.3 / m=0.5
pH=5.9 / IS=0.11 / [C]=9.8 / m=0.7
pH=5.9 / IS=0.11 / [C]=9.9 / m=0.9
0.40
0.20
pH=6.0 / IS=0.01 / [C]=9.4 / m=0.3
pH=6.0 / IS=0.02 / [C]=9.3 / m=0.2
pH=5.9 / IS=0.02 / [C]=9.8 / m=0.8
pH=5.9 / IS=0.02 / [C]=9.9 / m=0.6
0.00
0.00
60 120 180 240 300 60 120 180 240 300
Sedimentation Time (min) Sedimentation Time (min)
Figure S6.
Sedimentation plots for nano-TiO
2
aggregates at pH 6 in high (0.1 M; left) and low
(0.01-0.02 M; right) IS treatments. NOM concentrations increase from the top panels to the bottom panels within a column. In individual panels, filled symbols indicate the results from duplicate bacterial exudate treatments, while open symbols are for duplicate SRFA treatments.
The legends indicate the final pH after 24 hours of exposure, IS (in M) and NOM (mg DOC L
-1
) concentrations, and the sedimentation rate (m; min
-1
) during the initial 120 minutes of sedimentation. Two replicates for each treatment are distinguished.
S13
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
1.00
0.80
0.60
0.40
0.20
0.00
High IS Treatments pH=7.4 / IS=0.11 / [C]=0.0 / m=3.0
pH=7.4 / IS=0.11 / [C]=0.0 / m=3.3
pH=7.3 / IS=0.11 / [C]=0.0 / m=3.8
pH=7.5 / IS=0.11 / [C]=0.0 / m=4.1
1.00
0.80
0.60
0.40
0.20
0.00
pH=7.4 / IS=0.11 / [C]=0.1 / m=3.1
pH=7.4 / IS=0.11 / [C]=0.1 / m=3.0
pH=7.3 / IS=0.11 / [C]=0.1 / m=3.9
pH=7.6 / IS=0.11 / [C]=0.1 / m=3.8
1.00
0.80
0.60
0.40
0.20
0.00
pH=7.4 / IS=0.11 / [C]=0.9 / m=2.7
pH=7.4 / IS=0.11 / [C]=0.7 / m=2.7
pH=7.3 / IS=0.11 / [C]=1.0 / m=5.5
pH=7.4 / IS=0.11 / [C]=1.0 / m=5.9
1.00
0.80
0.60
0.40
0.20
0.00
pH=7.4 / IS=0.11 / [C]=4.7 / m=2.5
pH=7.4 / IS=0.11 / [C]=4.7 / m=2.4
pH=7.3 / IS=0.11 / [C]=4.9 / m=2.8
pH=7.5 / IS=0.11 / [C]=4.9 / m=2.2
1.00
0.80
0.60
0.40
0.20
0.00
Low IS Treatments pH=7.5 / IS=0.02 / [C]=0.0 / m=3.0
pH=7.5 / IS=0.02 / [C]=0.0 / m=3.2
pH=7.3 / IS=0.02 / [C]=0.0 / m=2.8
pH=7.5 / IS=0.02 / [C]=0.0 / m=3.2
pH=7.5 / IS=0.02 / [C]=0.1 / m=3.0
pH=7.5 / IS=0.02 / [C]=0.1 / m=2.8
pH=7.3 / IS=0.02 / [C]=0.1 / m=1.4
pH=7.5 / IS=0.02 / [C]=0.1 / m=0.9
pH=7.5 / IS=0.02 / [C]=0.9 / m=0.8
pH=7.4 / IS=0.02 / [C]=0.7 / m=1.3
pH=7.3 / IS=0.02 / [C]=1.0 / m=0.5
pH=7.5 / IS=0.02 / [C]=1.0 / m=0.6
pH=7.5 / IS=0.02 / [C]=4.7 / m=0.3
pH=7.4 / IS=0.02 / [C]=4.7 / m=0.2
pH=7.3 / IS=0.02 / [C]=4.9 / m=0.5
pH=7.5 / IS=0.02 / [C]=4.9 / m=0.4
1.00
0.80
0.60
1.00
0.80
0.60
0.40
0.20
pH=7.4 / IS=0.11 / [C]=9.3 / m=2.3
pH=7.4 / IS=0.11 / [C]=9.4 / m=2.4
pH=7.5 / IS=0.11 / [C]=9.9 / m=2.1
pH=7.5 / IS=0.11 / [C]=9.7 / m=3.0
0.40
0.20
pH=7.5 / IS=0.02 / [C]=11.3 / m=0.3
pH=7.4 / IS=0.02 / [C]=9.4 / m=0.2
pH=7.3 / IS=0.02 / [C]=9.9 / m=0.5
pH=7.5 / IS=0.02 / [C]=9.9 / m=0.5
0.00
0.00
60 120 180
Sedimentation Time (min)
240 300 60 120 180
Sedimentation Time (min)
240 300
Figure S7.
Sedimentation plots for nano-TiO
2
aggregates at pH 7.5 in high (0.1 M; left) and low (0.01-0.02 M; right) IS treatments. NOM concentrations increase from the top panels to the bottom panels within a column. In individual panels, filled symbols indicate the results from duplicate bacterial exudate treatments, while open symbols are for duplicate SRFA treatments.
The legends indicate the final pH after 24 hours of exposure, IS (in M) and NOM (mg DOC L
-1
) concentrations, and the sedimentation rate (m; min
-1
) during the initial 120 minutes of sedimentation. Two replicates for each treatment are distinguished.
S14
S.3 R
EFERENCES
Bhardwaj, R., Fang, X., Somasundaran, P., and Attinger, D. (2010). Self-assembly of colloidal particles from evaporating droplets: Role of DLVO interactions and proposition of a phase diagram. Langmuir , 26, 7833–7842.
Eshel, G., Levy, G., Mingelgrin, U., and Singer, M. (2004). Critical evaluation of the use of laser diffraction for particle-size distribution analysis. Journal of the Soil Science Society of
America , 68, 736–743.
Kim, A.S., and Yuan, R. (2005). Hydrodynamics of an ideal aggregate with quadratically increasing permeability. Journal of Colloid and Interface Science , 285, 627–633.
Li, X-Y., and Logan, B.E. (2001). Permeability of fractal aggregates. Water Research , 35, 3373–
3380.
Ramaswamy, S. (2001). Issues in the statistical mechanics of steady sedimentation. Adv. in Phys,
50, 297-341.
Ritchie, J.D., and Perdue, E.M. (2003). Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochimica et Cosmochimica Acta , 67,
85–96.
Seders, L.A., and Fein, J.B. (2011). Proton binding of bacterial exudates determined through potentiometric titrations. Chemical Geology , 285, 115–123.
Simon-Deckers, A., Loo, S., Mayne-L’hermite, M., Herlin-Boime, N., Menguy, N., Reynaud, C.,
Gouget, B., and Carrie ̀re, M. (2009). Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria.
Environmental Science & Technology , 43, 8423–8429.
S15