OXYGEN EQUILIBRIUM AND TRANSPORT
advertisement
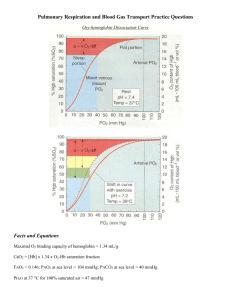
OXYGEN EQUILIBRIUM AND TRANSPORT CHAPTER 8 HOW DOES BLOOD CARRY OXYGEN? • MIXED VENOUS BLOOD ENTERS THE PULMONARY CAPILLARY WITH A PO2 OF ABOUT 40 mmHg AND IS EXPOSED TO AN ALVEOLAR PO2 OF ABOUT 100 mmHg. OXYGEN DIFFUSES DOWN THIS PRESSURE GRADIENT INTO THE BLOOD UNTIL EQUILIBRIUM IS ESTABLISHED. LIQUID(PLASMA) AND CELLULAR (ERYTHROCYTES) COMPONENTS OF BLOOD CARRY OXYGEN. OXYEN IS DISSOLVED IN PLASMA OR COMBINED WITH HEMOGLOBIN IN THE ERYTHROCYTE. OXYGEN DISSOLVED IN PLASMA • THE RELATIONSHIP BETWEEN PARTIAL PRESSURE AND DISSOLVED OXYGEN IS DIRECT AND LINEAR. • IN NORMAL ARTERIAL BLOOD WITH PaO2 = 100mmHg, THERE IS ABOUT 0.3 ml/dl OF DISSOLVED OXYGEN. • IF AN INDIVIDUAL BREATHES PURE OXYGEN, THE PaO2 INCREASES TO APPROXIMATELY 670 mmHg, THE DISSOLVED OXYGEN WILL INCREASE TO ABOUT 2.0 ml/dl • SOMEONE BREATHING PURE OXYGEN IN A HYPERBARIC CHAMBER AT 3 ATM WOULD CARRY NEARLY 6.5 ml /dl OF DISSOLVED OXYGEN. THIS AMOUNT IS ENOUGH TO SUPPLY MOST TISSUE NEEDS BY ITSELF. • DISSOLVED PLASMA OXYGEN CONTENT AT ANY PO2 IS CALCULATED AS FOLLOWS DISSOLVED OXYGEN = PaO2 X 0.003 ( HENRY’S LAW) OXYGEN COMBINED WITH HEMOGLOBIN HEMOGLOBIN COMBINED WITH OXYGEN • MOST OXYGEN IS TRANSPORTED IN CHEMICAL COMBINATION WITH Hb IN THE ERYTRHROCYTES. • WHEN Hb IS NOT CARRYING OXYGEN, THE FE2+ ION ATTACHED TO IT HAS FOUR UNPAIRED ELECTRONS. IN THIS DEOXYGENATED STATE,THE MOLECULE EXHIBITS THE CHARACTERISTICS OF A WEAK ACID. AS SUCH DEOXYGENATED Hb SERVES AS AN IMPORTANT BLOOD BUFFER FOR HYDROGEN IONS, A FACTOR CRITICALLY IMPORTANT IN CO2 TRANSPORT. • WHEN Hb IS CARRYING OXYGEN, ALL THE ELECTRONS OF THE IRON ION BECOME PAIRED AND Hb IS CONVERTED INTO ITS OXYGENATED STATE OXYHEMOGLOBIN. OXYGEN COMBINED WITH HEMOGLOBIN HEMOGLOBIN COMBINED WITH OXYGEN • HEMOGLOBIN-OXYGEN CARRYING CAPACITY - ONE GRAM OF Hb CAN CARRY ABOUT 1.34 ml OF OXYGEN - AVERAGE BLOOD HB CONTENT = 15 G/ DL Hb CARRYING CAPACITY = 1.34 X 15 = 20.1 ml/dl COMPARE WITH THE BLOOD’S ABILITY TO CARRY DISSOLVED OXYGEN DISSOLVED OXYGEN = 100 mmHg X 0.003 = 0.3 ml/dl HEMOGLOBIN SATURATION AND OXYGEN PARTIAL PRESSURE SATURATION IS A MEASURE OF THE PROPORTION OF AVAILABLE Hb THAT IS ACTUALLY CARRYING OXYGEN Hb SATURATION WITH O2 VARIES WITH CHANGES IN PaO2. UNLIKE DISSOLVED O2, Hb SATURATION IS NOT LINEARLY RELATED TO PaO2. INSTEAD,THE RELATIONSHIP FORMS AN S-SHAPED CURVE. ARTERIAL O2 SATURATION(SaO2) IS ABOUT 97.5% AT A NORMAL PO2 OF100 mmHg. ONLY 75%OF THE Hb IN MIXED VENOUS BLOOD IS HBO2. MIXED VENOUS O2 SATURATION (SvO2) IS THEREFORE NORMALLY 75%,CORRESPONDING WITH A PvO2 OF ABOUT 40 mmHg. IF Hb CONCENTRATION IS LOW (AS IN ANEMIA), THE BLOOD O2 CONTENT IS LOW, ALTHOUGH THE Hb PRESENT IS 100% SATURATED WITH O2. HEMOGLOBIN SATURATION AND OXYGEN PARTIAL PRESSURE LIKEWISE, LOW SATURATION DOES NOT AUTOMATICALLY MEAN THAT THE BLOOD O2 CONTENT IS BELOW NORMAL, ALTHOUGH THIS IS GENERALLY THE MEANING. FOR EXAMPLE, Hb CONCENTRTION MAY BE ABNORMALLY HIGH (POLYCYTHEMIA) IN INDIVIDUALS WHO HAVE CHRONIC HYPOXIA, THUS CAUSING THE BLOOD O2 CONTENT TO BE NORMAL, ALTHOUGH THE Hb SATURATION IS LOW. HEMOGLOBIN CAPACITY FOR OXYGEN • TOTAL OXYGEN CONTENT OF THE BLOOD THE TOTAL OXYGEN CONTENT OF THE BLOOD EQUALS THE SUM OF THAT DISSOLVED AND CHEMICALLY COMBINED WITH HEMOGLOBIN. CaO2 = ( 0.003 X PaO2 ) + ( Hb X 1.34 X SaO2) OXYHEMOGLOBIN EQUILIBRIUM CURVE PHYSIOLOGICAL ADVANTAGES OF THE HBO2 CURVE • THE OXYHEMOGLOBIN DISSOCIATION CURVE DEMONSTRATE THE EFFECTS OF OXYGEN LOADING (ASSOCIATION) AND UNLOADING (DISSOCIATION) IN THE LUNGS AND TISSUES. • POINT A REPRESENTS FRESHLY ARTERIALIZED BLOOD LEAVING THE LUNGS, WITH A PaO2 OF APPROXIMATELY 100 mmHg AND SO2 OF APPROXIMATELY 97%. • POINT V REPRESENTS PERFUSION OF BLOOD OF BODY TISSUES,OXYGEN UPTAKE CAUSES A FALL IN BOTH PaO2 ( APPROXIMATELY 40 mmHg) AND SaO2 (APPROXIMATELY 73%) Normal Loading and Unloading of Oxygen PHYSIOLOGICAL ADVANTAGES OF THE HBO2 CURVE • NORMAL LOADING AND UNLOADING OF OXYGEN • THE DIFFERENCE BETWEEN THE ARTERIAL AND VENOUS OXYGEN CONTENTS IS NORMALLY ABOUT 5 ml/dl. THE C(a-v)O2 IS THE AMOUNT OF OXYGEN GIVEN UP BY EVERY 100 ml OF BLOOD ON EACH PASS THROUGH THE TISSUES. TISSUES EXTRACT ABOUT 5 ml OF OXYGEN FROM EACH 100 ml OF ARTERIAL BLOOD. • THE C(a-v)O2 INDICATES OXYGEN EXTRACTION IN PROPORTION TO BLOOD FLOW. • THE PRINCIPLE RELATING C(a-v)O2 TO PERFUSION IS USED TO MONITOR TISSUE OXYGENATION AT THE BEDSIDE. HbO2 CURVE SHIFTS EFFECTS OF PCO2, pH, TEMPERATURE, AND 2,3 DPG • THE DECREASED AFFINITY OF HEMOGLOBIN FOR OXYGEN, OR THE RIGHT CURVE SHIFT CAUSED BY HIGH PCO2, IS KNOWN AS THE BOHR EFFECT. • CARBAMINOHEMOGLOBIN(CO2 COMBINED WITH Hb). INCREASED CARBAMINOHEMOGLOBIN DECREASES Hb’S O2 AFFINITY, SHIFTING THE HbO2 CURVE TO THE RIGHT. • IN HYPOTHERMIA THE HBO2 CURVE SHIFTS TO THE LEFT, AND MORE O2 REMAINS ATTACHED TO Hb. AT 20ºC, Hb IS 100% SATURATED AT A PO2 OF 60 mmHg. ALTHOUGH THIS GREATLY REDUCES O2 RELEASE TO THE TISSUES, HYPOTHERMIC TISSUES REQUIRE LESS O2. CLINICAL SIGNIFICANCE OF CHANGES IN Hb’S OXYGEN AFFINITY • EFFECT OF DECREASED Hb AFFINITY FOR O2 ON SaO2 AND PaO2 IN THE TISSUES. TUBE A REPRESENT BLOOD IN SYSTEMIC CAPILLARIES AS IF pH AND PCO2 DID NOT CHANGE. TUBE B SHOWS WHAT ACTUALLY ACCURS IN SYSTEMIC CAPILLARIES (PCO2 RISES AND pH DECREASES) THUS Hb’S O2 AFFINITY DECREASES,RELEASING O2 INTO THE PLASMA. PO2 IN PLASMA INCREASES AUGMENTING THE DIFFUSION GRADIENT INTO THE TISSUES. OXYGEN DELIVERY TO THE TISSUES • PaO2 AND SaO2 ALONE DO NOT PROVIDE AN ADEQUATE ASSESSMENT OF THE PATIENT’S OXYGENATION. THE PaO2 CAN BE 100 mmHg AND THE SaO2 98%, AND YET THE ANEMIC PATIENT WITH 5 g/dl OF Hb SUFFERS SERIOUS TISSUE HYPOXIA. • EVEN WITH A NORMAL Hb, PaO2 AND SaO2, A PERSON CAN STILL SUFFER FROM INADEQUATE OXYGEN DELIVERY IF THE BLOOD FLOW ( i.e. CARDIAC OUTPUT = Q) IS INADEQUATE. THUS THE TENDENCY TO EVALUATE THE OXYGENATION STATUS AS NORMAL JUST BECAUSE PaO2 AND SaO2 ARE NORMAL MUST BE RESISTED. • IN SUMMARY, FACTORS THAT AFFECT OXYGEN DELIVERY TO THE TISSUES INCLUDE: 1- Hb CONCENTRATION 2- SaO2 3CARDIAC OUTPUT (SaO2 AND PaO2 DETERMINE CaO2. OXYGEN DELIVERY DEPENDS ON CaO2 AND Q). CYANOSIS • WHEN THE Hb MOLECULE RELEASES O2 AND BECOMES DEOXYHEMOGLOBIN(DESATURATED Hb ), IT CHANGES IT SHAPE AND TURNS DEEP PURPLE. SEVERELLY HYPOXIC PEOPLE MAY HAVE ENOUGH DESATURATED Hb IN THEIR BLOOD THAT THE SKIN, NAILBEDS, LIPS, AND MUCOUS MEMBRANES APPEAR BLUE OR BLUE-GRAY. • MOST OBSERVERS DO NOT PERCEIVE CYANOSIS UNTIL THE AVERAGE DESATURATED Hb CONCENTRATION IN THE CAPILLARIES IS AT LEAST 5 g PER 100 ml ( 5 g/dl). • TWO TYPES OF CYANOSIS EXIST: PERIPHERAL AND CENTRAL CYANOSIS. PERIPHERAL IS CAUSED BY EXCESSIVELY LOW SvO2 WHILE SaO2 MAY BE NORMAL. • CYANOSIS DOES NOT ALWAYS ACCOMPANY SEVERE HYPOXEMIA, AND CYANOSIS IS SOMETIMES PRESENT IN THE ABSENCE OF HYPOXEMIA. HEMOGLOBIN ABNORMALITIES • CARBOXYHEMOGLOBIN • THE Hb MOLECULE HAS AN AFFINITY FOR CARBON MONOXIDE 210 TIMES GREATER THAN ITS AFFINITY FOR O2. THIS MEANS THAT BREATHING AIR (21%O2) AND 0.1% CO PUTS O2 AND CO ON EQUAL FOOTING IN COMPETING FOR Hb BINDING SITES. • CO NOT ONLYDECREASES THE AMOUNT OF Hb AVAILABLE FOR O2 TRANSPORT, IT ALSO IMPAIRS Hb’S RELEASE OF O2 AT THE TISSUES. • FETAL HEMOGLOBIN • METHEMOGLOBIN • SICKLE CELL HEMOGLOBIN