Document
advertisement

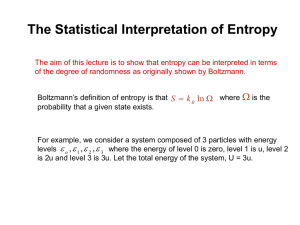
The Story of Spontaneity and Energy Dispersal You never get what you want: 100% return on investment Spontaneity Spontaneous process are those that occur naturally. Hot body cools A gas expands to fill the available volume A spontaneous direction of change is where the direction of change does not require work to bring it about. Spontaneity The reverse of a spontaneous process is a nonspontaneous process Confining a gas in a smaller volume Cooling an already cool object Nonspontaneous processes require energy in order to realize them. Spontaneity Note: Spontaneity is often interpreted as a natural tendency of a process to take place, but it does not necessarily mean that it can be realized in practice. Some spontaneous processes have rates sooo slow that the tendency is never realized in practice, while some are painfully obvious. Spontaneity The conversion of diamond to graphite is spontaneous, but it is joyfully slow. The expansion of gas into a vacuum is spontaneous and also instantaneous. 2ND LAW OF THERMODYNAMICS Physical Statements of the 2nd Law of Thermodynamics Kelvin Statements “No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work” “It is impossible for a system to undergo a cyclic process whose sole effects are the flow of an amount of heat from the surroundings to the system and the performance of an equal amount of work on the surroundings.” “It is impossible for a system to undergo a cyclic process that turns heat completely into work done on the surroundings.” Clausius statement It is impossible for a process to occur that has the sole effect of removing a quantity of heat from an object at a lower temperature and transferring this quantity of heat to an object at a higher temperature. Heat cannot flow spontaneously from a cooler to a hotter object if nothing else happens The 2nd Law of Thermodynamics The 2nd Law of Thermodynamics recognizes the two classes of processes, the spontaneous and nonspontaneous processes. Implications of the 2nd Law No heat engine can have an efficiency as great as unity No macroscopic process can decrease the entropy of the universe Are you kidding me? Thermodynamic Cat Hot Reservoir Heat Cold Reservoir Engine Heat Work I approve! What determines the direction of spontaneous change? The total internal energy of a system does NOT determine whether a process is spontaneous or not. Per the First Law, energy is conserved in any process involving an isolated system. What determines the direction of spontaneous change? Instead, it is important to note that the direction of change is related to the distribution of energy. Spontaneous changes are always accompanied by a dispersal of energy. Energy Dispersal Superheroes with energy blasts and similar powers as well as the Super Saiyans are impossible characters. They seem to violate the Second Law of Thermodynamics! Power Genki dama Energy Dispersal A ball on a warm floor can never be observed to spontaneously bounce as a result of the energy from the warm floor Energy Dispersal In order for this to happen, the thermal energy represented by the random motion and vibrations of the floor atoms would have to be spontaneously diverted to accumulate into the ball. Energy Dispersal It will also require the random thermal motion to be redirected to move in a single direction in order for the ball to jump upwards. This redirection or localization of random, disorderly thermal motion into a concerted, ordered motion is so unlikely as to be virtually impossible. Energy Dispersal and Spontaneity Spontaneous change can now be interpreted as the direction of change that leads to the dispersal of the total energy of an isolated system! INDEED! Entropy A state function, denoted by S. While the First Law can be associated with U, the Second Law may be expressed in terms of the S Entropy and the Second Law The Second Law can be expressed in terms of the entropy: The entropy of an isolated system increases over the course of a spontaneous change: ΔStot > 0 Where Stot is the total entropy of the system and its surroundings. Entropy: A molecular look Boltzmann formula: 𝑆 = 𝑘 ln 𝑊 Entropy is a reflection of the microstates, the ways in which the molecules of a system can be arranged while keeping the total energy constant. Entropy A simple definition of entropy is that it is a measure of the energy dispersed in a process. For the thermodynamic definition, it is based on the expression: Entropy For a measurable change between two states, In order to calculate the difference in entropy between two states, we find a reversible pathway between them and integrate the energy supplied as heat at each stage, divided by the temperature. Example Change in entropy of the surroundings: ΔSsur If we consider a transfer of heat dqsur to the surroundings, which can be assumed to be a reservoir of constant volume. The energy transferred can be identified with the change in internal energy dUsur is independent of how change brought about (U is state function Can assume process is reversible, dUsur= dUsur,rev Since dUsur = dqsur and dUsur= dUsur,rev, ∴ dqsur must equal dqsur,rev That is, regardless of how the change is brought about in the system, reversibly or irreversibly, we can calculate the change of entropy of the surroundings by dividing the heat transferred by the temperature at which the transfer takes place. Change in entropy of the surroundings: ΔSsur For adiabatic change, qsur = 0, so DSsur = 0 Entropy as a State Function To prove entropy is a state function we must show that ∫dS is path independent Sufficient to show that the integral around a cycle is zero or dS Sadi Carnot (1824) devised cycle to represent idealized engine Hot Th Reservoir Step 1: Isothermal reversible expansion @ Th qh w3 -w1 Step 2:Adiabatic expansion Th to Tc -w2 Step 3:Isothermal reversible compression @ Tc (sign of q negative) Engine w4 qc Cold Tc Reservoir Step 4: Adiabatic compression Tc to Th dq T 0 Carnot Engine How is that possible? Carnot Cycle Carnot Cycle Step 1: ΔU=0 Step 2: ΔU=w Step 3: ΔU=0 Step 4: ΔU=-w Carnot Cycle - Thermodynamic Temperature Scale The efficiency of a heat engine is the ratio of the work performed to the heat of the hot reservoir e=|w|/qh The greater the work the greater the efficiency Work is the difference between the heat supplied to the engine and the heat returned to the cold reservoir w = qh -(-qc) = qh + qc Therefore, e = |w|/qh = ( qh + qc)/qh = 1 + (qc/qh ) Hot Reservoir qh w Heat Engine Work Cold Reservoir Heat -qc Efficiency of Heat Engines Efficiency is the ratio of the work done by an engine in comparison to the energy invested in the form of heat for all reversible engines 𝑤 𝑞ℎ − 𝑞𝑐 𝑇ℎ − 𝑇𝑐 𝑇𝑐 e 𝑜𝑟 η = = = =1− 𝑞ℎ 𝑞ℎ 𝑇ℎ 𝑇ℎ All reversible engines have the same efficiency irrespective of their construction. Carnot Cycle - Thermodynamic Temperature Scale Hot William Thomson (Lord Kelvin) defined a substance-independent temperature scale based on the heat transferred between two Carnot cycles sharing an isotherm Reservoir qh He defined a temperature scale such that qc/qh = Tc/Th Or as Tc approaches 0 e approaches 1 Efficiency can be used as a measure of temperature regardless of the working fluid Applies directly to the power required to maintain a low temperature in refrigerators Heat Engine Work Cold Reservoir e = 1 - (Tc/Th ) Zero point on the scale is that temperature where e = 1 w Efficiency Heat -qc is maximized Greater temperature difference between reservoirs The lower Tc, the greater the efficiency Refrigeration Coefficient of performance (COP or β or c) 𝑞𝑐 𝑞𝑐 𝑇𝑐 𝐶𝑂𝑃 = = = 𝑤 𝑞ℎ − 𝑞 𝑐 𝑇ℎ − 𝑇𝑐 COP describes the qc in this case as the minimum energy to be supplied to a refrigeration-like system in order to generate the required entropy to make the system work. Entropy changes: Expansion Entropy changes in a system are independent of the path taken by the process ΔS = 𝑛𝑅 𝑉2 𝑙𝑛 𝑉1 Total change in entropy however depend on the path: Reversible process: ΔStot = 0 Irreversible process: ΔStot > 0 Isothermal Isochoric Isobaric Adiabatic ΔU 0 nCvΔT q+w w q nRT ln 𝑓 𝑉𝑖 or -wirrev nCvΔT nCpΔT or –wirrev 0 𝑉 wrev -nRT ln 𝑉𝑓 𝑉𝑖 0 -nRT ln 𝑉𝑓 𝑉𝑖 wirrev -pextΔV 0 -pextΔV ΔH 0 (for ideal gas) ΔU =ΔU + pΔV =nCpΔT ΔS = 𝑛𝑅 𝑙𝑛 𝑉𝑓 𝑉𝑖 = 𝑇2 𝐶𝑣𝑑𝑇 𝑇1 𝑇 = 𝑇2 𝐶𝑝𝑑𝑇 𝑇1 𝑇 𝑛𝑅∆𝑇 1−γ =-nCvΔT =-pextΔV 0 Entropy changes: Phase Transitions ΔtransS = ΔtransH Ttrans Trouton’s rule: An empirical observation about a wide range of liquids providing approximately the same standard entropy of vaporization, around 85 J/mol K. General equations for entropy during a heating process S as a function of T and V, at constant P 𝑇𝑓 𝑉𝑓 Δ𝑆 = 𝑛𝐶𝑝 𝑙𝑛 + 𝑛𝑅 ln 𝑇𝑖 𝑉𝑖 S as a function of T and P, at constant V 𝑇𝑓 𝑃𝑓 Δ𝑆 = 𝑛𝐶𝑣 𝑙𝑛 − 𝑛𝑅 ln 𝑇𝑖 𝑃𝑖 Measurement of Entropy (or molar entropy) Measurement of Entropy (or molar entropy) The terms in the previous equation can be calculated or determined experimentally The difficult part is assessing heat capacities near T = 0. Such heat capacities can be evaluated via the Debye extrapolation Measurement of Entropy (or molar entropy) In the Debye extrapolation, the expression below is assumed to be valid down to T=0. 𝐶𝑝 = 3 𝑎𝑇 Third Law of Thermodynamics At T = 0, all energy of thermal motion has been quenched, and in a perfect crystal all the atoms or ions are in a regular, uniform array. The localization of matter and the absence of thermal motion suggest that such materials also have zero entropy. This conclusion is consistent with the molecular interpretation of entropy, because S = 0 if there is only one way of arranging the molecules and only one microstate is accessible (the ground state). Third Law of Thermodynamics The entropy of all perfect crystalline substances is zero at T = 0. Nernst heat theorem The entropy change accompanying any physical or chemical transformation approaches zero as the temperature approaches zero: ΔS 0 as T 0 provided all the substances involved are perfectly crystalline. Third-Law entropies These are entropies reported on the basis that S(0) = 0. Exercises HELMHOLTZ AND GIBBS ENERGIES Clausius inequality 𝑑𝑞 𝑑𝑆 ≥ 𝑇 The Clausius inequality implies that dS ≥ 0. “In an isolated system, the entropy cannot decrease when a spontaneous change takes place.” Criteria for spontaneity 𝑑𝑞 𝑑𝑆 − ≥0 𝑇 In a system in thermal equilibrium with its surroundings at a temperature T, there is a transfer of energy as heat when a change in the system occurs and the Clausius inequality will read as above: Criteria for spontaneity When energy is transferred as heat at constant volume: 𝑑𝑞 𝑑𝑆 − ≥0 𝑇 *dq = dU 𝑇𝑑𝑆 ≥ 𝑑𝑈 At either constant U or constant S: Which leads to 𝑑𝑆𝑈, 𝑉 ≥ 0 𝑑𝑈𝑆, 𝑉 ≤ 0 𝑑𝑈 − 𝑇𝑑𝑆 ≤ 0 Criteria for spontaneity When energy is transferred as heat at constant pressure, the work done is only expansion work and we can obtain 𝑇𝑑𝑆 ≥ 𝑑𝐻 At either constant H or constant S: 𝑑𝑆𝐻 , 𝑝 ≥ 0 𝑑𝐻𝑆, 𝑝 ≤ 0 Which leads to 𝑑𝐻 − 𝑇𝑑𝑆 ≤ 0 Criteria for spontaneity We can introduce new thermodynamic quantities in order to more simply express 𝑑𝑈 − 𝑇𝑑𝑆 ≤ 0 and 𝑑𝐻 − 𝑇𝑑𝑆 ≤ 0 Helmholtz and Gibbs energy Helmholtz energy, A: Gibbs energy, G: A = U - TS G = H - TS dA = dU – TdS dG = dH – TdS dAT,V ≤ 0 dGT,p ≤ 0 Helmholtz energy A change in a system at constant temperature and volume is spontaneous if it corresponds to a decrease in the Helmholtz energy. Aside from an indicator of spontaneity, the change in the Helmholtz function is equal to the maximum work accompanying a process. Helmholtz energy , useful Variation of the Gibbs free energy with temperature Variation of the Gibbs free energy with pressure Variation of the Gibbs free energy with pressure Homework 1. When 1.000 mol C6H12O6 (glucose) is oxidized to carbon dioxide and water at 25°C according to the equation C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l), calorimetric measurements give ΔrHθ= -2808 kJ mol-1 and ΔrSθ = +182.4 J K-1 mol-1 at 25°C. How much of this energy change can be extracted as (a) heat at constant pressure, (b) work? 2. How much energy is available for sustaining muscular and nervous activity from the combustion of 1.00 mol of glucose molecules under standard conditions at 37°C (blood temperature)? The standard entropy of reaction is +182.4 J K-1 mol-1. 3. Calculate the standard reaction Gibbs energies of the following reactions given the Gibbs energies of formation of their components a) Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) b) C12H22O11(s) + 12 O2(g) 12 CO2(s) + 11 H2O(l) One for the road Life requires the assembly of a large number of simple molecules into more complex but very ordered macromolecules. Does life violate the Second Law of Thermodynamics? Why or why not?