Analogies among Mass, Heat, and Momentum Transfer
advertisement

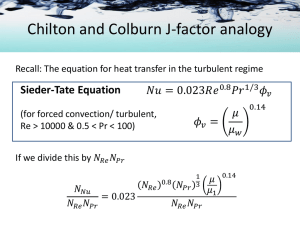
Analogies among Mass, Heat, and Momentum Transfer Analogies Heat Mass (sometimes) Momentum Analogies are useful tools 1. An aid to understand transfer phenomena 2. A sound means to predict behavior of systems for which limited quantitative data are available Molecular Transport Equations RECALL: driving force rate of transport = resistance d(v x ) yx dy MOMENTUM Newton’s law qy A d( c p T) dy HEAT Fourier’s law dcA J DAB dy * Ay MASS Fick’s law Analogous quantities in transport phenomena Reynolds Analogy The general transport equation can be written in the form 𝑑Γ 𝜓 =− 𝛿+𝐸 𝑑𝑥 where ψ = flux of a property at any value of x δ = molecular diffusivity E = eddy diffusivity Г = volume concentration of transferent property Turbulent diffusion equations Transfer coefficient for momentum In cylindrical geometry, 𝑑Γ 𝜓 =− 𝛿+𝐸 𝑑𝑟 Integrating the above equation and multiplying by A to get a rate equation, 𝛿+𝐸 𝜓𝐴 = −4 (Γ1 − Γ)𝐴 𝛾𝐷 where A = cross-sectional area perpendicular to flow 𝐸 = mean eddy diffusivity Γ −Γ 𝛾 = 1 = ratio of the difference in concentration of transferent property Γ1 −Γ0 between the wall and the mean value and the mean value of the fluid to the maximum difference between the wall and the center D = diameter Transfer coefficient for momentum 𝛿+𝐸 𝜓𝐴 = −4 (Γ1 − Γ)𝐴 𝛾𝐷 The transfer coefficient is then defined as 𝛿+𝐸 𝜑 = −4 𝛾𝐷 Substituting and rearranging, Γ1 − Γ 𝜓𝐴 = − 1 𝜑𝐴 Transfer coefficient for momentum 𝛿+𝐸 𝜓𝐴 = −4 (Γ1 − Γ)𝐴 𝛾𝐷 The transfer coefficient is then defined as 𝛿+𝐸 𝜑 = −4 𝛾𝐷 Substituting and rearranging, Γ1 − Γ 𝜓𝐴 = − 1 𝜑𝐴 Transfer coefficient for momentum Γ1 − Γ 𝜓𝐴 = − 1 𝜑𝐴 For momentum transfer, 𝜓=𝜏 Γ = 𝜌𝑣 𝜏 = −𝜑 [𝜌𝑣1 − 𝜌𝑣] Transfer coefficient for momentum 𝜏 = −𝜑 [𝜌𝑣1 − 𝜌𝑣] At the wall, v1 = 0 so that, 𝜏 = 𝜑 𝜌𝑣 𝜏 𝜑= 𝜌𝑣 If we divide by 𝑣, 𝑓 𝜑 𝜏 = = 2 2 𝑣 𝜌𝑣 The Reynolds analogy For turbulent transport, For heat transfer, For momentum transfer, We assume that α and μ/ρ are negligible, and that 𝛼𝑡 = 𝜖𝑡 The Reynolds analogy Dividing the momentum equation by the heat equation then gives 𝜏 𝑞 𝑐𝑝 𝐴 𝑇 𝑑𝑇 = 𝑇𝑖 𝑣𝑎𝑣 0 𝑑𝑣 The Reynolds analogy 𝑞 𝐴 2 Substituting = ℎ 𝑇 − 𝑇𝑖 and 𝜏𝑠 = 𝑓𝑣𝑎𝑣 𝜌/2 The Reynolds analogy Stanton number 𝑁𝑁𝑢 f 𝑁𝑆𝑡 = = 𝑁𝑅𝑒 𝑁𝑃𝑟 2 Dimensionless Groups Dim. Group Ratio Equation Prandtl, Pr molecular diffusivity of momentum / molecular diffusivity of heat Schmidt, Sc momentum diffusivity/ mass diffusivity Lewis, Le thermal diffusivity/ mass diffusivity Stanton, St heat transferred/ thermal capacity 𝑐𝑃 𝜇 𝑘 ν 𝐷𝐴𝐵 𝛼 𝐷𝐴𝐵 ℎ 𝑐𝑝 𝜌𝑣 The Reynolds analogy f h = 2 cp 𝜌 𝑣 Experimental results show that the above equation 1. Correlate data approximately for gases in turbulent flow 2. DOES NOT correlate experimental data for liquids in turbulent flow 3. DOES NOT correlate experimental data for any fluids in laminar flow * 0.6 < NPr for gases < 2.5 It was concluded that the Reynolds analogy is valid ONLY at NPr = 1 The Reynolds analogy In a similar manner, we can relate mass transfer with momentum transfer For turbulent transport And the complete Reynolds analogy is The Reynolds analogy f 𝑘𝑐′ = 2 𝑣 Experimental results show that the above equation 1. Correlate data approximately for gases in turbulent flow 2. DOES NOT correlate experimental data for liquids in turbulent flow 3. DOES NOT correlate experimental data for any fluids in laminar flow * NSc for gases ~ 1.0 It was concluded that the Reynolds analogy is valid ONLY at NSc = 1 The Reynolds analogy CONCLUSIONS 1. At NPr = NSc = 1, the mechanisms for mass, heat, and momentum are identical 2. For other fluids, transfer processes differ in some manner functionally related to the Pr and Sc numbers. The Reynolds analogy Note that the Reynolds analogy assumes that 1. the turbulent diffusivities are equal and 2. the molecular diffusivities are negligible. When are these assumptions not valid? 1. For other fluids, where 𝑁𝑃𝑟 ≠ 𝑁𝑆𝑐 ≠ 1 usually the case for liquids 2. We CANNOT neglect molecular diffusivities in the boundary layer where diffusion, conduction, and viscosity are important