Electrochemistry MAE-212
advertisement
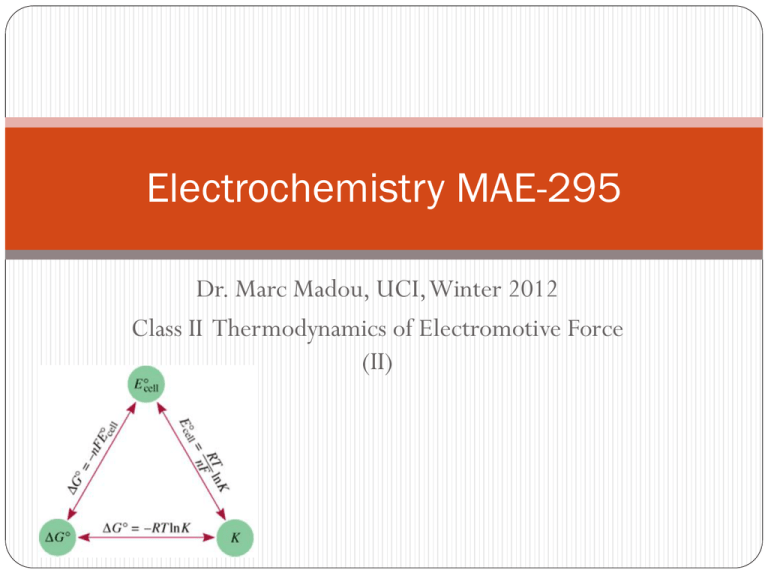
Electrochemistry MAE-295 Dr. Marc Madou, UCI, Winter 2012 Class II Thermodynamics of Electromotive Force (II) Table of Content Standard Redox Potentials Table Thermodynamics Ecell, ΔG, and Keq Derivation of the Nernst Equation Some example problems Structure of the solid/electrolyte interface Standard Redox Potentials Table • E0 is for the reaction as written • The more positive E0 the greater the tendency for the substance to be reduced • The half-cell reactions are reversible • The sign of E0 changes when the reaction is reversed • Changing the stoichiometric coefficients of a half-cell reaction does not change the value of E0 (Intrinsic vs extrinsic properties) Relation between Equilibrium constant, Gibbs free energy and EMF of a cell The free energy function is the key to assessing the way in which a chemical system will spontaneously evolve. In the Gibbs-Duhem formalism of the Gibbs free energy we can write: constant T constant P don’t change shape don’t stretch it Relation between Equilibrium constant, Gibbs free energy and EMF of a cell The chemical potential or electrochemical potential (if we are dealing with a charged particle) is the measure of how all the thermodynamic properties vary when we change the amount of the material present in the system. Formally we can write: Integration of the expression for the dependence of amount of material on the Gibbs function, leads to the following relationship [Homework I]: Relation between Equilibrium constant, Gibbs free energy and EMF of a cell Every substance has a unique propensity to contribute to a system’s energy. We call this property Chemical Potential: m When the substance is a charged particle (such as an electron or an ion) we must include the response of the particle to an electrical field in addition to its Chemical Potential. We call this the Electrochemical Potential (F is the Faraday constant, z the charge on the particle and f the potential): m= m + z F f Relation between Equilibrium constant, Gibbs free energy and EMF of a cell • Start with the First Law of Thermodynamics and some standard thermodynamic relations and we find: •And therefore, the Gibbs function is at the heart of electrochemistry, for it identifies the amount of work we can extract electrically from a system. Relation between Equilibrium constant, Gibbs free energy and EMF of a cell • Now we can easily see how this Gibbs function relates to a potential. • By convention, we identify work which is negative with work which is being done by the system on the surroundings. And negative free energy change is identified as defining a spontaneous process. • Note how a measurement of a cell potential directly calculates the Gibbs free energy change for the process. Relation between Equilibrium constant, Gibbs free energy and EMF of a cell The propensity for a given material to contribute to a reaction is measured by its activity, a. How “active” is this substance in this reaction compared to how it would behave if it were present in its standard state? • Activity scales with concentration or partial pressure. a C/C˚ (solution) and a P/P˚ (gas) BUT… • intermolecular interactions • deviations from a direct correspondence with pressure or concentration Relation between Equilibrium constant, Gibbs free energy and EMF of a cell Definition of activity is then: • Activity coefficients close to 1 for dilute solutions and low partial pressures. • Activity changes with concentration, temperature, other species, etc. Can be very complex. • Generally, we ignore activity coefficients for educational simplicity, but careful work always requires its consideration. Relation between Equilibrium constant, Gibbs free energy and EMF of a cell How does chemical potential change with activity? Integration of the expressions for the dependence of amount of material on the Gibbs function, leads to the following relationship [home-work I]: Relation between Equilibrium constant, Gibbs free energy and EMF of a cell • How does Gibbs free energy change with concentration/activity? Same dependence as for the chemical potential: • When we apply this to a reaction, the reaction quotient comes into to play, giving us: • Say we have the reaction : Relation between Equilibrium constant, Gibbs free energy and EMF of a cell The above reaction is a generic reaction and in order to analyze this chemical process mathematically, we formulate the reaction quotient Q: • It always has products in the numerator and reactants in the denominator • It explicitly requires the activity of each reaction participant. • Each term is raised to the power of its stoichiometric coefficient. Relation between Equilibrium constant, Gibbs free energy and EMF of a cell- Nernst Equation Take the expression for the Gibbs dependence on activity and rewrite this in terms of cell potential: • The relation between cell potential E and free energy gives: • Rearrange and obtain the Nernst Equation: Relation between Equilibrium constant, Gibbs free energy and EMF of a cell- Nernst Equation The equation is often streamlined by restricting discussion to T = 25 °C and inserting the values for the constants, R and F. • Note the difference between using natural logarithms and base10 logarithms. • Be aware of the significance of “n” – the number of moles of electrons transferred in the process according to the stoichiometry chosen. Relation between Equilibrium constant, Gibbs free energy and EMF of a cell Chemical Equilibrium • The reaction proceeds, Q changes, until finally DG=0. At that moment the overall reaction stops. This is equilibrium. • This special Q* (the only one for which we achieve this balance) is renamed Keq, the equilibrium constant. Relation between Equilibrium constant, Gibbs free energy and EMF of a cell -Chemical Equilibrium When the system is at equilibrium, the proper quotient of equilibrium concentrations is equal to the equilibrium constant: The system is at equilibrium when the concentrations are in their equilibrium values, so [A] = [A]e, [B] = [B]e, etc. and thus: Q* = Keq Relation between Equilibrium constant, Gibbs free energy and EMF of a cell Chemical Equilibrium Example problems: Cu is cathode (it is reduced). Zn is anode (it is oxidized). • Note that n=2 for this reaction. =1 =1 • Activity for solid materials is 1; replace activities with concentrations. Example problems: What is the potential in the cell if [Cu2+] = 0.01 M and [Zn2+] = 1.00 M? • Note that the cell potential decreased by about 60mV. This was a change in concentration of TWO orders of magnitude, but since it was also a TWO electron process, we saw the same 60 mV change in potential. Example Problems: Nernst equation demonstrates that potential depends upon concentration. A cell made of the same materials, but with different concentrations, will also produce a potential difference. Cu | Cu2+ (0.001 M) || Cu2+ (1.00 M) | Cu What is standard cell potential E˚ for this cell? What is the cell potential E? What is “n”, the number of electrons transferred? Which electrode, anode or cathode, will be in numerator? Example Problems: The equations we have derived allow us to relate measured cell potentials to Standard Gibbs Free Energies of reaction. These in turn are related to a reaction’s equilibrium constant. Consider the cell Pt | I– (1.00 M), I2 (1.00 M) || Fe2+ (1.00 M), Fe3+ (1.00 M) | Pt Standard Cell Potential is (from tables) = 0.771 V - 0.536 V = +0.235 V This is the free energy change. It leads to the equilibrium constant for the reaction. Example Problems: Fe2+ + 2e– Fe -0.44 Sn2+ + 2e– Sn V2+ + 2e– V -1.19 Ag+ + e– Ag To get a final positive cell potential, the more negative half-reaction (V) must act as the anode. Fe2+ + V Fe + V2+ Ecell = -0.44 - (-1.19) = +0.75 V -0.14 +0.80 More negative potential reaction is the anode. Multiply the Ag reaction by 2, but don’t modify the cell potential. 2 Ag+ + Sn 2 Ag + Sn2+ Ecell = +0.80 - (-0.14) = +0.94 V Example Problems: A fuel cell is an electrochemical cell that requires a continuous supply of reactants to keep functioning Anode: Cathode: 2H2 (g) + 4OH- (aq) 4H2O (l) + 4e- O2 (g) + 2H2O (l) + 4e- 4OH- (aq) 2H2 (g) + O2 (g) 2H2O (l) Example Problems: Corrosion Example Problems: Cathodic Protection of an Iron Storage Tank Example Problems: Electrolysis is the process in which electrical energy is used to cause a non-spontaneous chemical reaction to occur. Example Problems: Electrolysis of Water Example Problems: Electrolysis and Mass Changes charge (Coulombs) = current (Amperes) x time (sec) 1 mole e- = 96,500 C = 1 Faraday How much Ca will be produced in an electrolytic cell of molten CaCl2 if a current of 0.452 A is passed through the cell for 1.5 hours? 2Cl- (l) Anode: Cathode: Ca2+ (l) + 2e- Cl2 (g) + 2eCa (s) Ca2+ (l) + 2Cl- (l) Ca (s) + Cl2 (g) 2 mole e- = 1 mole Ca mol Ca = 0.452 C s = 0.0126 mol Ca = 0.50 g Ca x 1.5 hr x 3600 s hr x 1 mol e96,500 C x 1 mol Ca 2 mol e- Structure of the solid/electrolyte interface Every solid/liquid has a interface that one would like to control --the better one can control that interface the better one can say something about the environment Interfaces are very complex often involving fractals (beach, trees, snow flakes, etc.) rather than smooth transitions, this implies , for example, that perfect selectivity will be hard to achieve (too many different binding sites) Structure of the solid/electrolyte interface Charge carriers in electrode materials: Metals (e.g. Pt) : electrons Semiconductors (e.g. n-Si) : electrons and holes Solid electrolytes (e.g. LaF3 ) : ions Insulators (e.g. SiO2):no charge carriers Mixed conductors (e.g. IrOx) : ions and electrons Solution (e.g. 1 M NaCl in H2O): solvated ions Double layer-(in case of a metal 10-40 µF cm-2) Inner Helmholtz plane (IHP) Outer Helmholtz plane (OHP) Gouy-Chapman layer (GCL)