Topic 16 Kinetics
advertisement
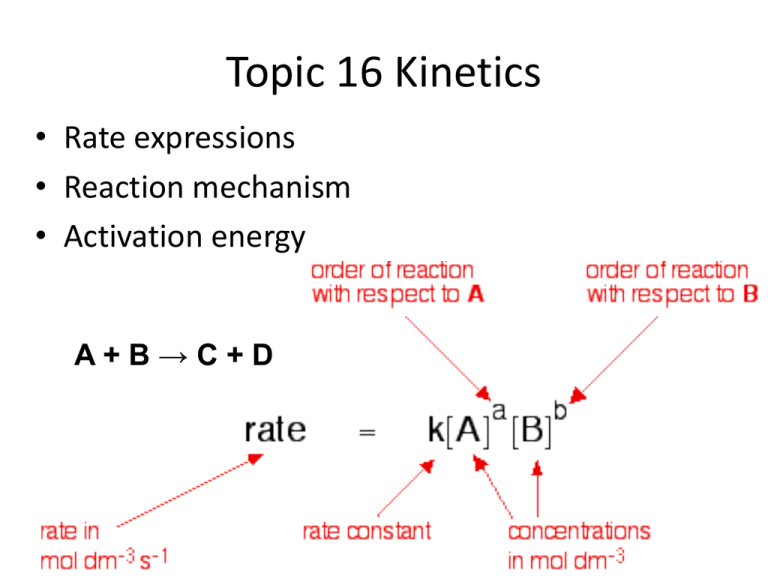
Topic 16 Kinetics • Rate expressions • Reaction mechanism • Activation energy A+B→C+D 16.1 Rate expression • Change in concentration usually affects the rate of reaction • The change in rate isn’t the same for all reactants (A and B) • Must be determined by experiment. (Change the concentrations of one reactant and hold the others constant) A+B→C+D rate =k*[A]a*[B]b If a reaction involves the reactants A, B etc => The Rate expression Rate of reaction = - d[A]/dt = k[A]a[B]b k = rate constant • Order of reaction: “a” in substance A and “b” in substance B • Overall order of reaction: a+b What happens to the rate in the reaction A+BC+… [A] [B] Reaction rate Double the concentration Keep constant 20 = 1 Double the concentration Keep constant No change X2 Double the concentration Keep constant X4 22 = 4 Order 21 = 2 Zero order First order Second order Reactants A, B and C in four experiments with altering concentrations: A + B + C => …… Experiment [B] mol*dm-3 1.600 [C] mol*dm-3 0.0600 mol*dm-3*s-1 1 [A] mol*dm-3 0.400 Initial rate 2 0.800 1.600 0.0600 9.72 3 0.400 0.800 0.0600 4.86 4 0.800 1.600 0.1800 87.5 4.86 Compare Experiment 1 and 2: Experiment [B] mol*dm-3 1.600 [C] mol*dm-3 0.0600 mol*dm-3*s-1 1 [A] mol*dm-3 0.400 2 0.800 1.600 0.0600 9.72 Initial rate: [A]: 2x [B] and [C] : constant 2X rate => [A]1 The reaction is first order in [A] Initial rate 4.86 Compare Experiment 1 and 3: Experiment [B] mol*dm-3 1.600 [C] mol*dm-3 0.0600 mol*dm-3*s-1 1 [A] mol*dm-3 0.400 3 0.400 0.800 0.0600 4.86 Initial rate: [A]: constant [B]: ½ [C] : constant same rate => [B]0 The reaction is zero order in [B] Initial rate 4.86 Compare Experiment 2 and 4: Experiment [B] mol*dm-3 1.600 [C] mol*dm-3 0.0600 mol*dm-3*s-1 2 [A] mol*dm-3 0.800 4 0.800 1.600 0.1800 87.5 Initial rate: [A]: constant [B]: constant [C] : 3X 32 = 9X=> [C]2 The reaction is second order in [C] Initial rate 9.72 Conclusion • Rate = k*[A]1’*[B]0*[C]2 = k*[A]1*[C]2 • Overall order 1+2 = 3 • k can be calculated using the data from one of the experiments above Exercises • 1-2 on page 120 The order can also be found in a graph where initial concentration is set against initial rate. The gradient of the graph => rate of the reaction. First order reactions They show an exponential decrease: the time to half the concentration is equal to go from ½ to 1/4 Half life, t½ = 0.693/k 16.2 Reaction mechanism Types of reactions: • A Products • A + B Products Molecularity Unimolecular Bimolecular • In a Bimolecular reaction the reactants collide and form an activated complex 2 molecules Nucleophilic Substitution bimolecular, SN2- topic 10 If we study the reaction: H CH3COCH3 + I2 CH3COCH2I + HI + • It could be a bimolecular process with a rate expression rate = k*[CH3COCH3] *[I2] • The rate is independent of [I2], but first-order in propanone and acid => rate = k*[CH3COCH3]*[H+] • The reaction must proceed through a series of steps, a mechanism must be found: + H H O O C + F a s t e q u ilib riu m C C H3 H3C C H3 H3C P ro p a n o n e H H O O + C C C H3 H3C C H3 H2C + H+ S lo w R a te D e te rm in in g S te p H O + O I2 C H2C C H3 C + C H3 H2C I HI Fast • CH3C(OH+)CH3 is known as a intermediate, not an activated complex, though it occur at an energy minimum. In the mechanism there will be several activated complexes H O + C H3C CH3 Intermediate Activated complex = Transition state, T O C O + H3C H 3 C CH3 C H2C C C H 3 I H O C O CH3 H2C H C H3 Exercises • 1 and 2 on page 122 16.3 Activation energy Recall: Maxwell-Boltzmann energy distribution curve. Temperature Average speed Higher temperature =>More particles with higher speed => Greater proportion of particles with energy enough to react The Arrhenius equation • The rate constant, k, can be given if collision rate and orientation is given • Ea = activation energy • T = temperature, K • R = Gas constant The equation can also be given in a logarithmic form: Exersize: Consider the following graph of ln k against (temperature in Kelvin) for the second order decomposition of N2O into N2 and O N2 O → N 2 + O (a) State how the rate constant, k varies with temperature, T (b) Determine the activation energy, Ea, for this reaction. (c) The rate expression for this reaction is rate = k [N2O]2 and the rate constant is 0.244 dm3 mol–1 s–1 at 750 °C. A sample of N2O of concentration 0.200 mol dm–3 is allowed to decompose. Calculate the rate when 10 % of the N2O has reacted. Solution: (a) State how the rate constant, k varies with temperature, T The Arrhenius equation (it’s in the Data booklet): k=Ae(-Ea/RT) Logaritming the equation on both sides: lnk=lnA –Ea/RT In the graph we see that the gradient is negative Answer: when k increases T decreases (b) Determine the activation energy, Ea, for this reaction The gradient (-Ea/R) can be calculated from the graph: DY/DX= -3/0,1*10-3= -3*104= -30000 Therefore: Ea=gradient*R=-30000*8.31= 2,49*105 Answer: Ea= 2,49*105 Solution: (c) The rate expression for this reaction is rate = k [N2O]2 and the rate constant is 0.244 dm3 mol–1 s–1 at 750 °C. A sample of N2O of concentration 0.200 mol dm–3 is allowed to decompose. Calculate the rate when 10 % of the N2O has reacted. 10 % has reacted → 90 % left → 0.200*0.9= 0.180 mol/dm3 Rate= 0.244 * [0.180]2 = 7.91*10-3