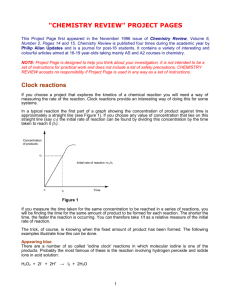
1.3 c) Solutions / Solution Stoichiometry

1.5 SOLUTIONS
*In a typical laboratory, the majority of reactions carried out are in _______________ rather than the gaseous phase.
Chemists need to make up solutions of known concentrations.
Definitions i) Solution : a ______________________ mixture of a ______________ that has been dissolved in a _______________
*When the solvent is water the solution is described as an _______________ solution. Solutions are often described as being concentrated or dilute . ii) Standard Solution or Primary Standard : a solution with a _________________ concentration; prepared using a
___________________ flask
Some Units of Concentration i) molarity
mol per unit volume (molar concentration
mol dm -3 )
the amount (in mol) of a substance dissolved in 1 dm 3 of solvent
concentration in mol dm -3 may also be referred to as ________________, and square brackets are sometimes used to denote molar concentration. Also, a 1.0
“molar”
solution = 1.0 mol dm -3 solution. e.g. ii) mass per unit volume (g dm -3 )
the amount (in grams) of a substance dissolved in 1 dm 3 of solvent iii) parts per million (ppm)
like %, but out of 1 million (10 6 ), instead of 100 e.g.
Extension: iv) molality mol per kilogram (mol kg -1 ) v) %(v/v) = %(m/v) = %(m/m) =
Concentration Calculations:
1.
Calculate the concentration, in mol dm -3 , of a solution formed when 0.475 g of magnesium chloride, MgCl
2
, is completely dissolved in water to make a solution with a volume of 100 cm 3 .
2.
Determine the concentration, in mol dm -3 , of chloride ions in question 1.
3.
Calculate the mass, in g, of potassium hydrogen phthalate, C
8
H
5
O
4
K (a primary standard) in 250 cm 3 of a 1.25 mol dm -3 solution.
4.
A standard solution is prepared by dissolving 5.30 g of sodium carbonate, Na
2
CO
3
, in 250 cm 3 of distilled water in a volumetric flask. A 10.0 cm 3 sample of this solution is removed by bulb pipette and diluted with water to the final volume 0.100 dm 3 . Calculate the concentration, in mol dm -3 , of the diluted solution.
Introduction to Titration
Definitions: i) titration : involves a standard solution of known concentration which is added to a solution of unknown concentration until the chemical reaction is complete ii) indicator : the reaction progress is monitored through colour changes using indicators iii) analyte: the substance that is being ______________ iv) titrant: the substance for which the concentration is ____________
Acid-Base Titration Calculations
(Neutralization Reaction Stoichiometry)
1.
It is found that 10.00 cm 3 of 0.2000 mol dm -3 aqueous sodium carbonate requires 25.00 cm 3 of hydrochloric acid to just neutralize it. Determine the concentration of the hydrochloric acid.
2.
Calculate the volume, in dm 3 , of 0.390 mol dm -3 potassium hydroxide, KOH, solution that will neutralize 25.0 cm 3 of 0.350 mol dm -3 sulfuric acid, H
2
SO
4
.
Pre-Lab (Preparing Standard Solutions) Task
Procedure #1 Dissolving a Known Mass of Solute
Outline how to prepare 100 cm 3 of 0.500 mol dm -3 lead(II) nitrate, Pb(NO
3
)
2
, using the solid solute.
Procedure #2 Diluting An Existing Standard Solution
Outline how to prepare a 2.0
dm 3 of 0.10
mol dm -3 sulfuric acid solution via the dilution of the 18 mol dm -3 , concentrated stock solution.