More reading materials on properties of pure substance (largely
advertisement

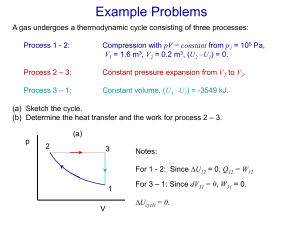
MEL140 Properties of pure substances Pure substance • A pure substance has the same chemical composition throughout. • Are the following confined in a fixed volume pure substances: – – – – – Ice (single component, single phase) A mixture of water and water vapor (single component, multiphase) Air in gas phase (multi-component, single phase) Oil in contact with water (multi-component single phase) A gaseous mixture containing N2,O2,H2O, CO2 obtained from burning kerosene (multicomponent, single phase) – Liquid air in contact with gaseous air (multicomponent, multiphase) Objective: evaluating the properties for single component pure substances existing in one or more phases (multiphase). Y Y Y N Y N Alert: in chemistry a pure substance is defined such that it consists of one component (chemical species) and therefore must be “non-mixture”. We follow a different definition (see above) in engineering thermodynamics. Phases A region within matter with distinct molecular arrangement that is homogeneous throughout that region which is separated from other regions (if any) by distinct boundary surfaces. Physical properties (like density and refractive index) of each phase is different. The three principal phases: Solid Liquid Gas http://www.chem.purdue.edu/gchelp/atoms/states.html Phase equilibrium • A system can be composed of subsystems with different molecular arrangements separated by phase boundaries (phases). • Phase equilibrium prevails when no transfer of mass happens between phases. The state postulate • A property is characteristic of the system such as specific volume (v), temperature (T), pressure (P), (specific) internal energy (u). • A state is the condition of a system as determined by its properties. • A simple compressible system is a system whose only mode of performing quasi-equilibrium work is through a change of its volume against a pressure. • The state postulate: The state of a simple compressible system consisting of a pure substance is completely specified by two independent intensive properties. • The state postulate can be represented by an equation of state such as f(p,v,T)=0 (or say g(p,v,u)=0). It is often convenient to represent this functional relationship by – A surface in p,v,T (or u,p,v) space or more commonly its projections on (p,v), (T,v) and (p,T) planes. – Tables of properties The P-v diagram 3 Remove just enough heat to keep temperature constant as the volume is reduced. It will be observed that except during 2-3, pressure also needs to be increased for executing this process in a quasi-equilibrium manner. During 1-2 you 4-5 (not during 2-3-4) 2 1 Shows isotherms on P-v diagram The critical state: recapitulation • At the critical state (Tc, Pc), saturated liquid and saturated vapor states are identical (SLL intersects SVL). • Increasing/decreasing pressure at a given temperature leads to condensation/evaporation only if a state lies below the critical isotherm. Phase change processes Critical properties of common fluids • Water/steam: • Refrigerant 134a or R134a or 1,1,1,2-Tetrafluoroethane in your “freeze”: • Nitrogen: – CP: 374o C (647.1 K), 22 Mpa (~more than 200 atm) at which specific volume 0.003106 m3/kg (~three times less dense than @ STP) – BP at atmospheric pressure (101.325 kPa): 100o C (1 atm) – – CP: 101o C, 4 Mpa (~40 atmosphere) BP at atmospheric pressure : -26o C, 101.325 kPa (1 atm) – CP: -147o C, 3.4 MPa – BP at atmospheric pressure : -196o C • Carbon-dioxide: • • – CP: 31.05oC ,7.39 Mpa (CO2 is not a “gas” in Delhi for six months, i.e. Apr-Sept) – BP at atmospheric pressure: -78.5oC Table A.1 (Tc,Pc,vc) How far a state is away from critical point? Curious facts: • • Critical isotherm and the gas-vapor nomenclature Supercritical fluidsT>Tcr and P>Pcr Principle of corresponding states (van der Waal, 1880) • Reduced temperature: Tr=T/Tc • Reduced pressure: Pr=P/Pc • Reduced volume: vr=v/vc Regardless of the substance, there is a universal equation of state connecting the reduced co-ordinates. So, thermodynamic states of different substances “correspond”. “my equation of state has a universal form which can be identified by its predicted behavior at critical point” Can be stated as: v r f ( Pr , T r ) “Other equation of states might also be given similar universal forms by the same procedure”. Principle of corresponding states (van der Waal, 1880, continued) • Correspondence means the same reduced co-ordinates should mean the sameness of a third reduced property such as reduced volume. • Compressibility factor is an important reduced property given by: Z PV RT • Z0 Pr V r Tr Z signifies departure from ideal gas behavior. More discussion on significance in notes. • Principle of corresponding states: All fluids when compared at the same Tr and Pr have the same Z and deviate from the ideal gas behavior to about the same degree. • This principle is the basis of classifying systematizing organizing and compacting experimental measurements on P, V and T. Critical compressibility of real gases Phase change processes Some terminology • Compressed liquid or sub-cooled liquid: Liquid which is not about to vaporize (State 1) • Saturated liquid: liquid which is about to vaporize (State 2) • Saturated vapor: vapor which is about to condense (State 4) • Saturated liquid-vapor mixture: a mixture of saturated liquid and saturated vapor (State 3) • Superheated vapor: vapor that is not about to condense (State 5) Phase change processes Compressed/ subcooled liquid Saturated vapor Saturated liquid Superheated vapor Saturated liquid vapor mixture Latent heat • The energy absorbed by a system during a phase change process at a given pressure/temperature is called latent heat. – Latent heat of fusion (melting) – Latent heat of vaporization (boiling) • Latent heat goes to change the molecular potential energy; in-fact temperature, a measure of molecular kinetic energy remains constant during a phase change process. Saturation temperature, saturation pressure and saturation curve • Phase change processes (e.g. “saturated liquid” boiling to “saturated vapor”) under a given pressure ( “saturation pressure” or Psat) take place at a given temperature ( “saturation temperature” or Tsat). • Therefore Psat=f (Tsat). A plot of this function is the saturation curve Saturation curve for water Property diagram for phase change processes: the T-v diagram. 234 Construct at different pressures The critical point The state (“point”) at which the saturated liquid and the saturated vapor states are identical. For water The T-v diagram: saturated liquid line and the saturated vapor line Shows isobars on T-v diagram Saturated liquid and saturated vapor lines meet at the critical point. The P-v diagram Remove weights to change pressure during 1-2, 4-5 (not during 2-3-4) Shows isotherms on P-v diagram Extending the P-v diagram to include solid phase a solid at temperature lower than melting point b solid begins melting c solid completely melted d liquid begins to vaporize e liquid completely vaporized a b c d e P-v diagram of a substance which contracts on freezing (most except water) Extending the P-v diagram to include solid phase a ice at -10oC, 1 atm Saturated liquid lines Saturated solid line LIQUID a c b d SOLID e b ice begins melting (0oC), 1 atm c ice completely melted (0oC), 1 atm d water begins to vaporize (100oC), 1 atm e water completely vaporized (100oC), 1 atm P-v diagram of a substance which expands on freezing (e.g. water) The triple line • The states where all three phases co-exist in equilibrium lie on a straight line on the P-V or T-v diagram known as a triple line. • All the “triple states” appear as a point on the p-T diagram and the corresponding (T,v) is called a “triple point”. • Triple point of water: (0oC, 0.61 kPa) The P-T diagram (“phase diagram”) The P-v-T surface For substances which contract on freezing For substances which expand on freezing (such as water). Enthalpy: a combination property • Enthalpy (h): – h=u+pv – Enthalpy is useful for studying processes (such as vaporization, heat transfer) taking place at constant pressure and processes that involve flow work Objective • Evaluate properties of states corresponding to: – saturated liquid and saturated vapor – saturated liquid-vapor mixtures – superheated vapor – compressed/sub-cooled liquid Saturated liquid and saturated vapor • Subscript f represents “saturated liquid state” • Subscript g represents “saturated vapor state” • Subscript i represents saturated solid state. • Propertyfg=PropertygPropertyf Specified From Table A-4 – e.g. v fg =vg-vf represents volume change on vaporization – hfg = =hg-hf represents the “latent heat” or enthalpy of vaporization. Saturated liquid and saturated vapor • Saturated states lie on the curve f(Psat, Tsat)=0 and can therefore be specified by specifying either Psat, or Tsat • Table A-4 for water: (Psat,vf,vg,vfg,uf,ug, ufg,hf,hg,hfg,sf,sg,sfg) listed against Tsat • Table A-5 for water: (Tsat,vf,vg,vfg,uf,ug, ufg,hf,hg,hfg,sf,sg,sfg) listed against Psat • Same data in Tables A-4 and A-5 From Table A-4 Saturated liquid and saturated vapor • Subscript f represents “saturated liquid state” • Subscript g represents “saturated vapor state” • Propertyfg=PropertygPropertyf – e.g. v represents volume change on vaporization – hfg = =hg-hf represents the “latent heat” or enthalpy of vaporization (for vaporization under constant pressure) From Table A-4 fg =vg-vf Saturated liquid-vapor mixtures • Refer to same Tables A-4 and A-5. • The proportion of saturated vapor in the mixture is indicated by a new property “quality” or “dryness fraction”: x m ass of saturated vapor total m ass of the m ixture mg m f mg • The average value of a specific extensive property y (such as v,u,h) etc. for the mixture can be calculated from y y f xy fg Saturated liquid-vapor mixtures • Refer to same Tables A-4 and A-5. • The proportion of saturated vapor in the mixture is indicated by a new property “quality” or “dryness fraction”: x m ass of saturated vapor total m ass of the m ixture • • • • • mg m f mg x=0 for saturated liquid 0<x<1 for saturated liquid-vapor mixture x=1 for saturated vapor x is undefined for compressed liquid and superheated vapor The average value of a specific extensive property y (such as v,u,h) etc. for the mixture can be calculated from y y f xy fg Superheated vapor • At least two properties need to be given to specify the state according to state postulate • Usually either T or P and another property is given: • At a superheated state: – – – – – P<Psat @ given T T> Tsat @ P v>vg @ P/T u>ug @ P/T h>hg @ P/T • Visit Table A-6 for water Properties of pure substances (continued) MEL140 Compressed liquid • At a compressed liquid state – P>Psat @ given T – T<Tsat @ given P – v<vf @ given P/T – u<uf @ given P/T – h<hf @ given P/T • Usually compressed liquid tables are not available except Table A-7 for water at P> 0.5 MPa Approximately evaluating properties at the compressed liquid state • For a compressed liquid, properties are weakly dependent on p. • Treat compressed liquid as a saturated liquid at the given temperature. • Evaluate: – – – – v'vf@T u'uf@T h'hf? Usually better approximation for h is: • h=u+pv'uf+pvf=hf-psatvf+pvf =hf+(p-psat)vf using v'vf and u'uf@T and hf=(uf+ psatvf). Evaluate compressed liquid v at (T,p) SteamNBS 105 SteamNBS 104 5 10 P [kPa] 103 104 100°C 102 101 10 T, At given v is not 10 8x10 sensitive to p. 10°C 0 -1 -4 3 P [kPa] 10 102 3x10-3 10-3 3 v [m /kg] 100°C 101 0 10 10-1 8x10-4 If no v is tabulated at given (P,T) find vf@T=Tsat using saturation table (Table A.4 for water) 10°C 10-2 10-1 100 3 v [m /kg] 101 102 103 Determining the state (summary) Saturated liquid-vapor mixture (A.4,A.5 for water) Compressed/subcooled liquid (A.7, A.4 if not A.7 for water) Superheated vapor (A.6 for water) P=Psat(T) P>Psat @ T P<Psat@T T=Tsat(P) T<Tsat @ P T>Tsat@T vf<v<vg v<vf@P/T v>vg@T uf<u<ug u<uf@P/T u>ug@P/T hf<h<hg h<hf@P/T h>hg@P/T x=(y-yf)/yfg where y=v/u/h (0<x<1) x undefined x undefined