Q11 Chem Tut 5 - 12S7F-note
advertisement

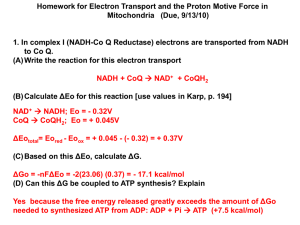
Presented by 12S7F Yu Tian :D 1 Problem statement >.< • Aluminium fluoride is made industrially by reacting aluminium oxide with hydrogen fluoride at a high temperature. Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2 Problem statement >.< Al2O3 (s) HF (g) AlF3 (s) H2O (g) ΔHfo / kJ mol-1 -1676 -271 -1504 -242 ΔSfo / J K-1 mol-1 -313 +7.0 -266 -44.4 UNIT: J K-1 mol-1 NOT kJ K-1 mol-1 3 Problem statement >.< (a) Use the data given to calculate: • The standard enthalpy change, ΔHo , of this reaction • The standard entropy change, ΔSo , of this reaction 4 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 5 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 6 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 7 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 8 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) By Hess’s Law, the enthalpy change of a particular reaction is determined only by the initial and final states of the system regardless of the pathway taken. 9 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 10 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 11 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 12 Problem wracking x.x Al2O3 (s) + 6HF (g) 2AlF3 (s) + 3H2O (g) 2Al (s) + 3H2 (g) + 3F2 (g) + O2 (g) 13 (b) Use the values calculated in (a) to calculate ΔGo . ΔGo = ΔHo – T x ΔSo = (-432) x 103 – (298) x (-394.2) J mol-1 = - 315 J mol-1 (to 3 s.f.) 14 Problem statement (c) How will the value of ΔGo for this reaction change with temperature? What consequences will this have for the conditions used to make AlF3 industrially? 15 Problem wracking (c) How will the value of ΔGo for this reaction change with temperature? GRADIANT OF THE GRAPH Formula: ΔGo = ΔHo – ΔSo x T ΔGo 0 Y- INTERCEPT ΔSo is negative T ΔHo 16 Problem wracking (c) How will the value of ΔGo for this reaction change with temperature? • Ans: As value of ΔSo is negative, according to formula Formula: ΔGo = ΔHo – ΔSo x T, the higher the reaction temperature, the more positive the value of ΔGo becomes. 17 Problem wracking (c) What consequences will this have for the conditions used to make AlF3 industrially? Sign of ΔGo Positive Reaction is not feasible. 0 The system is at equilibrium Negative Reaction is feasible 18 Problem wracking (c) What consequences will this have for the conditions used to make AlF3 industrially? From ΔGo = - 315 J mol-1 - Under standard condition, the reaction is not spontaneous/feasible For the reaction to be feasible, ΔGo > 0 T > 1100 K or T> 823 ℃ - High temperature is needed in industrial production of AlF3 19 20