Carnot cycle
advertisement
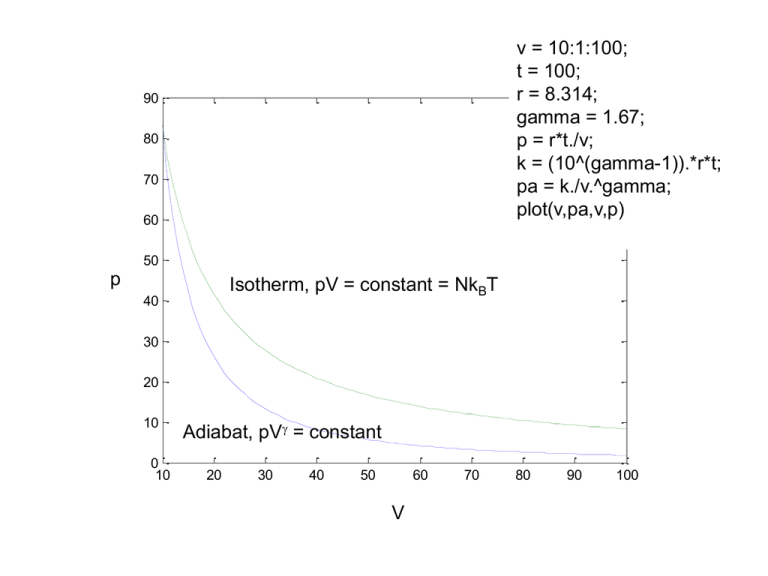
v = 10:1:100; t = 100; r = 8.314; gamma = 1.67; p = r*t./v; k = (10^(gamma-1)).*r*t; pa = k./v.^gamma; plot(v,pa,v,p) 90 80 70 60 50 p Isotherm, pV = constant = NkBT 40 30 20 10 0 10 Adiabat, pV = constant 20 30 40 50 60 V 70 80 90 100 Carnot cycle v = 10:1:100; th = 100; tl = 50; r = 8.314; gamma = 1.67; p1 = r*th./v; k1 = (30^(gamma-1)).*r*th; pa1 = k1./v.^gamma; p2 = r*tl./v; k2 = (30^(gamma-1)).*r*tl; pa2 = k2./v.^gamma; plot(v,p1,v,pa1,v,p2,v,pa2) Carnot cycle Carnot cycle pA, VA, TA Isotherm 1 Th p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Isotherm 2 pC, VC, TC V Carnot cycle pA, VA, TA Isotherm 1 Th p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Isotherm 2 pC, VC, TC V Carnot cycle pA, VA, TA Isotherm 1 Tl p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Ql Isotherm 2 pC, VC, TC V Carnot cycle pA, VA, TA Isotherm 1 Tl p Qh pB, VB, TB Adiabat 2 Adiabat 1 pD, VD, TD Ql Isotherm 2 pC, VC, TC V Why such a strange engine? Will discuss in class Efficiency of a Carnot engine 𝑝𝐴 , 𝑉𝐴 , 𝑇𝐴 Isotherm 1 ⇒ 𝑇𝐴 = 𝑇𝐵 = 𝑇ℎ p 𝑝𝐵 , 𝑉𝐵 , 𝑇𝐵 Adiabat 2 Adiabat 1 𝑝𝐷 , 𝑉𝐷 , 𝑇𝐷 Isotherm 2 ⇒ 𝑇𝐶 = 𝑇𝐷 = 𝑇𝑙 V 𝑝𝐶 , 𝑉𝐶 , 𝑇𝐶 Efficiency of a Carnot engine 𝑝𝐴 , 𝑉𝐴 , 𝑇ℎ Isotherm 1 p ⇒ Δ𝑄ℎ = 𝑅𝑇ℎ ln 𝑉𝐵 𝑉𝐴 Adiabat 2 𝑝𝐵 , 𝑉𝐵 , 𝑇ℎ ⇒ Δ𝑄 = 0 𝛾−1 𝛾−1 ⇒ 𝑇𝑙 𝑉𝐷 = 𝑇ℎ 𝑉𝐴 Adiabat 1 ⇒ Δ𝑄 = 0 𝛾−1 𝛾−1 ⇒ 𝑇ℎ 𝑉𝐵 = 𝑇𝑙 𝑉𝐶 𝑝𝐷 , 𝑉𝐷 , 𝑇𝑙 Isotherm 2 𝑉𝐷 ⇒ Δ𝑄𝑙 = 𝑅𝑇𝑙 ln 𝑉𝐶 V 𝑝𝐶 , 𝑉𝐶 , 𝑇𝑙 Efficiency of a Carnot engine 𝑉𝐵 Δ𝑄ℎ = 𝑅𝑇ℎ ln 𝑉𝐴 𝛾−1 𝑇ℎ 𝑉𝐵 𝛾−1 = 𝑇𝑙 𝑉𝐶 𝑉𝐷 Δ𝑄𝑙 = 𝑅𝑇𝑙 ln 𝑉𝐶 𝛾−1 𝑇𝑙 𝑉𝐷 (1) From isotherm 1 (2) From adiabat 1 (3) From isotherm 2 𝛾−1 = 𝑇ℎ 𝑉𝐴 (4) From adiabat 2 From the first law of thermodynamics: Δ𝑈 = Δ𝑄 + Δ𝑊 For the complete Carnot cycle Δ𝑈 = 0 since 𝑈 is a state variable Efficiency of a Carnot engine 𝑉𝐵 Δ𝑄ℎ = 𝑅𝑇ℎ ln 𝑉𝐴 𝛾−1 𝑇ℎ 𝑉𝐵 𝛾−1 = 𝑇𝑙 𝑉𝐶 𝑉𝐷 Δ𝑄𝑙 = 𝑅𝑇𝑙 ln 𝑉𝐶 𝛾−1 𝑇𝑙 𝑉𝐷 (1) From isotherm 1 (2) From adiabat 1 (3) From isotherm 2 𝛾−1 = 𝑇ℎ 𝑉𝐴 (4) From adiabat 2 From the first law of thermodynamics: Δ𝑈 = Δ𝑄 + Δ𝑊 For the complete Carnot cycle Δ𝑈 = 0 since 𝑈 is a state variable ⇒ Δ𝑄 = −Δ𝑊 Δ𝑊is the work done on the engine (system), let 𝑊 be the work done by the engine ⇒ 𝑊 = −Δ𝑊 = Δ𝑄 𝑉 𝑉 From (1) and (3): Δ𝑄 = Δ𝑄ℎ +Δ𝑄𝑙 = 𝑅𝑇ℎ ln 𝐵 + 𝑅𝑇𝑙 ln 𝐷 𝑉 𝑉 𝐴 𝐶 Efficiency of a Carnot engine 𝑉𝐵 Δ𝑄ℎ = 𝑅𝑇ℎ ln 𝑉𝐴 𝛾−1 𝑇ℎ 𝑉𝐵 (1) From isotherm 1 𝛾−1 = 𝑇𝑙 𝑉𝐶 (2) From adiabat 1 𝑉𝐷 Δ𝑄𝑙 = 𝑅𝑇𝑙 ln 𝑉𝐶 𝛾−1 𝑇𝑙 𝑉𝐷 (3) From isotherm 2 𝛾−1 = 𝑇ℎ 𝑉𝐴 (4) From adiabat 2 𝑉 𝑉 From (1) and (3): Δ𝑄 = Δ𝑄ℎ +Δ𝑄𝑙 = 𝑅𝑇ℎ ln 𝐵 + 𝑅𝑇𝑙 ln 𝐷 = 𝑊 𝑉 𝑉 𝐴 Efficiency is defined as: 𝐶 Output Input Output is the work done by the engine i.e. 𝑊 and input is the heat absorbed by the engine i.e. Δ𝑄ℎ ⇒ 𝜂 efficiency = 𝑊 Δ𝑄ℎ +Δ𝑄𝑙 Δ𝑄𝑙 = =1+ Δ𝑄ℎ Δ𝑄ℎ Δ𝑄ℎ Efficiency of a Carnot engine 𝑉𝐵 Δ𝑄ℎ = 𝑅𝑇ℎ ln 𝑉𝐴 𝛾−1 𝑇ℎ 𝑉𝐵 (1) From isotherm 1 𝛾−1 = 𝑇𝑙 𝑉𝐶 (2) From adiabat 1 𝑉𝐷 Δ𝑄𝑙 = 𝑅𝑇𝑙 ln 𝑉𝐶 𝛾−1 𝑇𝑙 𝑉𝐷 (3) From isotherm 2 𝛾−1 = 𝑇ℎ 𝑉𝐴 ⇒ 𝜂 efficiency = (4) From adiabat 2 𝑊 Δ𝑄ℎ +Δ𝑄𝑙 Δ𝑄𝑙 = =1+ Δ𝑄ℎ Δ𝑄ℎ Δ𝑄ℎ 𝑉𝐷 𝑉𝐷 ln 𝑇𝑙 𝑉𝐶 𝑉𝐶 ⇒𝜂 = 1+ =1− 𝑉 𝑇ℎ ln 𝑉𝐴 𝑅𝑇ℎ ln 𝑉𝐵 𝑉𝐵 𝐴 𝑅𝑇𝑙 ln 𝑇ℎ 𝑉𝐶 ⇒ = (2) 𝑇𝑙 𝑉𝐵 𝛾−1 and (4) 𝑇ℎ 𝑉𝐷 ⇒ = 𝑇𝑙 𝑉𝐴 (from (1) and (3)) 𝛾−1 𝑉𝐶 𝑉𝐷 ⇒ = 𝑉𝐵 𝑉𝐴 𝑉𝐴 𝑉𝐷 ⇒ = 𝑉𝐵 𝑉𝐶 Efficiency of a Carnot engine 𝑇𝑙 ⇒𝜂 =1− 𝑇ℎ Laws of thermodynamics 0. There is a game 1. You can never win 2. You cannot break even, either 3. You cannot quit the game Carnot engine: Schematic representation 𝑇ℎ 𝑄ℎ = Δ𝑄ℎ 𝑊 = Δ𝑄ℎ +Δ𝑄𝑙 = 𝑄ℎ − 𝑄𝑙 Carnot 𝑄𝑙 = Δ𝑄𝑙 = −Δ𝑄𝑙 𝑇𝑙 Carnot engine: Schematic representation 𝑇ℎ 𝑄ℎ 𝑊 = 𝑄ℎ − 𝑄𝑙 Carnot 𝑄𝑙 𝑇𝑙 Carnot engine is reversible 𝑇ℎ 𝑄ℎ 𝑊 = 𝑄ℎ − 𝑄𝑙 Carnot 𝑄𝑙 𝑇𝑙 Carnot engine is reversible (refrigerator) 𝑇ℎ 𝑄ℎ 𝑊 = 𝑄ℎ − 𝑄𝑙 Carnot 𝑄𝑙 𝑇𝑙 Carnot’s theorem reversible Of all heat engines working between two given temperatures, none is more efficient than a Carnot engine Carnot engine is reversible (refrigerator) 𝑇ℎ 𝑄ℎ′ 𝑄ℎ 𝑊 ′ = 𝑄ℎ′ − 𝑄𝑙′ 𝑊 = 𝑄ℎ − 𝑄𝑙 R Carnot 𝑄𝑙′ 𝑄𝑙 𝑇𝑙 Adjust the cycles so that 𝑊 = 𝑊 ′ Carnot engine is reversible (refrigerator) 𝑇ℎ 𝑄ℎ′ 𝑄ℎ 𝑊 = 𝑄ℎ − 𝑄𝑙 = 𝑄ℎ′ − 𝑄𝑙′ R Carnot 𝑄𝑙′ 𝑄𝑙 𝑇𝑙 Carnot engine is reversible (refrigerator) 𝑇ℎ 𝑄ℎ′ 𝑄ℎ 𝑊 𝑊 > 𝑄ℎ′ 𝑄ℎ 𝑊 = 𝑄ℎ − 𝑄𝑙 = 𝑄ℎ′ − 𝑄𝑙′ ⇒ 𝑄ℎ′ < 𝑄ℎ ⇒ 𝑄ℎ − 𝑄ℎ′ > 0 R Carnot If 𝜂′ > 𝜂 then: Also: 𝑄ℎ − 𝑄𝑙 = 𝑄ℎ′ − 𝑄𝑙′ 𝑄𝑙′ 𝑄𝑙 𝑇𝑙 ⇒ 𝑄ℎ − 𝑄ℎ′ = 𝑄𝑙 − 𝑄𝑙′ > 0 Is this possible? 𝑇ℎ 𝑄ℎ 𝑄ℎ − 𝑄ℎ′ 𝑄ℎ′ ⇒ 𝑄ℎ − 𝑄ℎ′ = 𝑄𝑙 − 𝑄𝑙′ > 0 𝑊 = 𝑄ℎ − 𝑄𝑙 = 𝑄ℎ′ − 𝑄𝑙′ R Carnot 𝑄𝑙 𝑄𝑙 − 𝑄𝑙′ 𝑇𝑙 ⇒ 𝑄ℎ > 𝑄ℎ′ 𝑎𝑛𝑑 𝑄𝑙 > 𝑄𝑙′ 𝑄𝑙′ The Second Law of Thermodynamics • Clausius’ statement: It is impossible to construct a device that operates in a cycle and whose sole effect is to transfer heat from a cooler body to a hotter body. ⇒ 𝜂′ ≯ 𝜂 Carnot’s theorem reversible Of all heat engines working between two given temperatures, none is more efficient than a Carnot engine 𝑊𝑖𝑟𝑟 < 𝑊𝑟𝑒𝑣 ⇒ 𝜂𝑖𝑟𝑟 < 𝜂𝑟𝑒𝑣 For reversible engines 𝑇ℎ 𝑄ℎ′ 𝑄ℎ 𝑊 = 𝑄ℎ − 𝑄𝑙 = 𝑄ℎ′ − 𝑄𝑙′ R Carnot 𝑄𝑙′ 𝑄𝑙 𝑇𝑙 ⇒ 𝜂′ ≯ 𝜂 𝑎𝑛𝑑 𝜂 ≯ 𝜂′ ⇒ 𝜂 = 𝜂′ Carnot’s theorem Of all heat engines working between two given temperatures, none is more efficient than a Carnot engine All reversible engines working between two temperatures have the same efficiency as 𝜂Carnot The Second Law of Thermodynamics • Clausius’ statement: It is impossible to construct a device that operates in a cycle and whose sole effect is to transfer heat from a cooler body to a hotter body. • Kelvin-Planck statement: It is impossible to construct a device that operates in a cycle and produces no other effect than the performance of work and the exchange of heat from a single reservoir. Carnot refrigerator and Kelvin violator 𝑇ℎ 𝑄ℎ′ 𝑄ℎ ⇒ 𝑄ℎ −𝑄ℎ′ = 𝑄𝑙 > 0 𝑊 = 𝑄ℎ′ = 𝑄ℎ − 𝑄𝑙 Kelvin violator Carnot 𝑄𝑙 𝑇𝑙 ⇒ 𝑄ℎ −𝑄𝑙 = 𝑄ℎ′ Carnot engine and Claussius violator 𝑇ℎ 𝑄ℎ 𝑄𝑙 𝑊 = 𝑄ℎ − 𝑄𝑙 Claussius violator Carnot 𝑄𝑙 𝑄𝑙 𝑇𝑙