Alkanes
advertisement

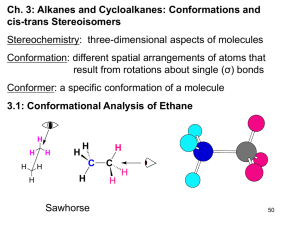
Alkanes Acyclic: CnH2n+2 Cyclic (one ring): CnH2n Bicyclic (two rings) : CnH2n-2 Only single bonds, sp3 hybridization, close to tetrahedral bond angles Physical properties • Boiling points – Lower than other organic molecules of same size. – Lower attractive forces between molecules than in alcohols. methane -164 oC water 100 oC hexane 68.7 oC 1-pentanol 137 oC Intermolecular Forces • Ionic Forces • Hydrogen Bonding Dispersion Forces: due to fluctuating motionStrength of the electrons in a molecule. Motion in one molecule is correlated with that in • other Dipole Dipole Forces the molecule. • Dispersion Forces Dispersion Forces and Molecular Structure Branching decreases surface area, reduces dispersion forces and, thus, boiling point. Molecular Structure and Heat of Combustion Difference in heats of combustion indicates a greater stability of branched structures. 18.8 kJ -5470.6 -5451.8 8CO2 + 9H2O Isomerism and Naming • Hexane CH3CH2CH2CH2CH2CH3 2-methylpentane CH3 CH3CH2CH2CHCH3 CycloAlkanes Cl 1-chloro-3-methylcyclohexane 1,2-diisopropylcyclobutane 1-methyl-2-propylcyclopropane Bicycloalkanes Parent name: name of alkane with same number of carbons. Number from bridgehead along largest bridge. If substituent choose bridgehead to assign low number to substituent. Size of bridges indicated by number of carbons in bridge. Examples of numbering 1 Cl 2 5 6-chlorobicyclo[3.1.1]heptane 7 2,7-dimethylbicyclo[4.2.2]decane Conformations • Rotations about single bonds produce different conformations. 60 Staggered Conformation. Eclipsed Conformation. Newman Projections Staggered Conformation. More stable! Eclipsed Conformation. Less stable. Rotational Profile of ethane CH3 CH3CH2CH2CHCH3 What are the forces in a molecular structure? Torsional strain: Strain between groups on adjacent atoms. A-B-C-D. Worst when eclipsed; best when staggered. Bond angle strain: when a bond angle, A-B-C, diverges from the ideal (180, 120, 109) View from here yields view below. View from here yields view below. Rotation about C2 – C3 in butane 120 deg. Anti conformation Methyls 180 deg, lower energy Gauche conformation, Methyls closer, 60 deg, more repulsion, higher energy H CH3 H H H H CH3 Anti!! Gauche!! H H H3C H CH3 Energy Profile for Rotation in Butane Three valleys (staggered forms) 120 apart; Three hills (eclipsed) 120 apart. Problem: Rotational profile of 2-methylbutane about C2-C3. First, staggered structures. 60 180 300 H H H Me H Me Me H H H Me Me Me Rotate the front Me group. Relative energies…. H Me Me Me H Now, eclipsed…. H Me H Me Me 240 H HH HMe H Me 180 120 0 Me H HH HMe H Me Me H Me Me This was the high energy staggered structure,180 deg. Shown for reference only. Now relative energies….. 360 = 0 Me Me H Me Me H Now put on diagram… HH HH Me Me HMe H Me H Me Me H Me Me H Me Me H H H H Me Me H Me Me 60 120 eclipsed Me H H H Me Me H Me Me Me staggered H H 0 HMe 180 240 300 360 Conformations of cycloalkanes: cyclopropane Planar ring (three points define a plane); sp3 hybrization: 109o. Hydrogens eclipsing. Torsional angle strain. Bond angle strain. Should be 109 but angle is 60o. Cyclopropane exhibits unusual reactivity for an alkane. Conformation of cyclobutane Fold on diagonal Planar: eclipsing, torsional strain and bond angles of 90o Folded, bent: less torsional strain but increased bond angle strain Cyclobutane molecular dynamics Cyclopentane Cyclohexane Boat conformation planar: bond angle 120, eclipsed. Ideal solution: Everything staggered and all angles tetrahedral. Chair conformation Chair Conformation Axial: Equatorial: Axial and Equatorial Axial Up/Equatorial Down: (A/E) Equatorial Up/Axial Down: (E/A) A/E E/A E/A A/E A/E E/A Ring Flips Chair Boat or A becomes E Twisted Boat E becomes A Up stays Up Down stays Down Chair Substituents: Axial vs Equatorial R R axial substituent equatorial substituent Substituent, R D G Preference for Equatorial K at 25 deg -CH3, methyl 7.28 kJ/mol 18.9 -CH2CH3, ethyl 7.3 19. -CH(CH3)2, iso propyl 9.0 38. -C(CH3)3, tert butyl 21.0 4.8 x 103 Substituent Interactions R H H Destabilizes axial substituent. Each repulsion is about 7.28/2 kJ = 3.6 kJ 1,3 diaxial repulsions Alternative description: R gauche interactions Each repulsion is still about 3.6 kJ. Note that the gauche interaction in butane is about 3.8. Newman Projection of methylcyclohexane Axial methyl group Equatorial methyl group gauche anti H CH3 ring H ring H ring ring CH3 H H H Disubstituted cyclohexanes 1,2 dimethylcyclohexane trans trans cis cis 7.3 kJ (axial) 3.6 kJ (gauche) 3.6 kJ (gauche) CH3 CH3 CH3 0.0 kJ equatorial ring ring CH CH3 3 CH CH3 3 ring ring 7.3 kJ (axial) H H H H 7.3 + 3.6 = 10.9 kJ H3C interactions 0.0 kJ equatorial ring ring H C H3 3C CH CH3 3 H H ring ring H H 7.3 + 3.6 = 10.9 kJ trans cis 7.3 kJ (axial) CH3 CH3 H3C 3.6 kJ (gauche) 0.0 kJ equatorial CH3 7.3 kJ (axial) diequatorial diaxial H ring H 3C CH3 ring CH3 H 0.0 kJ + 3.6 kJ = 3.6 kJ ring H H ring CH3 14.6 kJ + 0.0 kJ = 14.6 kJ When does the gauche interaction occur? carbons adjacentcarbons onadjacent beon mustbe groupsmust alkylgroups alkyl axial / equatorial axial/ equatorial equatorial equatorial equatorial/ /equatorial axial axial axial/ /axial Translate ring planar structure into E/A 3D A/E A/E C(CH3)3 Assume the tert-butyl group is equatorial. E/A E/A A/E C(CH3)3 Energy accounting No axial substituents One 1,2 gauche interaction between methyl groups, 3.6 kJ/mol Total: 3.6 kJ Problem: Which has a higher heat of combustion per mole, A or B? A B t-Bu t-Bu E/A E/A E/A E/A E/A E/A A/E A/E 3.6 3.6 7.2 3.6 7.3 7.3 18.2 More repulsion, higher heat of combustion by 11.0 kJ/mol Trans and Cis Decalin decahydronaphthalene decalin bicyclo [4.4.0] decane Now build cis decalin, both same side. Build trans decalin starting from cyclohexane, one linkage up, one down Trans sites used on the left ring Trans sites used on the right ring Trans decalin Locked, no ring flipping Cis sites used on left ring. Cis sites used on right ring. Cis decalin, can ring flip Trans fusions determine geometry A/E H3C E/A HO E/A H A/E What is the geometry of the OH and CH3? E/A A/E H Trans fusions, rings must use equatorial position for fusion. Rings are locked. The H’s must both be axial Work out axial / equatorial for the OH and CH3. OH is equatorial and CH3 is axial