Solute
advertisement

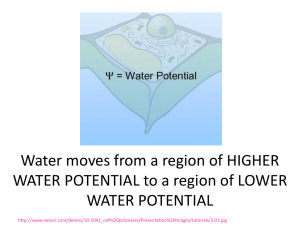
Solution • a homogeneous mixture of two or more components. The components of a solution are atoms, ions, or molecules, which makes them 10-9 m or smaller in diameter. • Ex. Salt water Suspensions • The particles in suspensions are larger than those found in solutions. Components of a suspension can be evenly distributed by a mechanical means, like by shaking the contents, but the components will settle out. • Ex: Oil and Water Colloids • Particles intermediate size can be mixed such that they remain evenly distributed without settling out. These particles range in size from 10-8 to 10-6 m in size. • The mixture they form is called a colloidal dispersion. • A colloidal dispersion consists of colloids in a dispersing medium. • Ex: Milk Parts of a solution: 1. Solute: substance being dissolved (NaCl) 2. Solvent: dissolving medium (H2O) Solvation: the process of solvent particles surrounding solute particles to form a solution “Like dissolves Like” This is the general rule to determine whether solvation occurs. “Like” refers to the solvent and solute being either polar or nonpolar. Polarity of a molecule is a result of how the electrons are shared and arranged. Water is a polar molecule. “Universal solvent” Telling Them Apart • You can tell suspensions from colloids and solutions because the components of suspensions will eventually separate. • Colloids can be distinguished from solutions using the Tyndall effect. – A beam of light passing through a true solution, such as air, is not visible. – Light passing through a colloidal dispersion will be reflected by the larger particles and the light beam will be visible. (Ex. smoky or foggy air) When Substances Combine: 1.Soluble: a substance that dissolves in a solvent 2.Insoluble: a substance that does not dissolve in a solvent 3.Immiscible: two liquids that are immiscible, they separate after mixing (water & oil) 4.Miscible: two liquids that are soluble in each other. Rate of Solvation Factors affecting the rate of solvation: 1. Agitation: stirring, shaking; allows particles to dissolve faster 2. Temperature: heat adds energy which allows particles to move faster and disassociate from each other faster. 3. Particle size: smaller particles increase surface area for the solvent to act on. Affect of Temperature on Solubility Solubility : maximum amount of solute that will dissolve in a given amount of solvent, 100g usually Solubility Terminology: 1.Saturated solution: contains the maximum amount (g) of dissolved solute 2.Unsaturated solution: contains LESS than the maximum amount (g) of dissolved solute 3.Supersaturated solution: contains MORE that the maximum amount of dissolved solute. What type of a solution is a solution that contains 70g PbNO3 at 40ºC in 100g H2O? SATURATED What type of a solution is a solution that contains 35g NaCl at 80ºC in 100g H2O? UNSATURATED What type of a solution is a solution that contains 120g K2NO3 at 60ºC in 100g H2O? SUPERSATURATED Factors Affecting Solubility: 1.Temperature: many substances are more soluble at high temperature. But, gases will dissolve better at colder temperatures. Factors Affecting Solubility: 2. Pressure: Gases will dissolve best under pressure. Henry’s Law: solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. “The BENDS” Deep sea divers may experience a condition called the "bends" if they do not readjust slowly to the lower pressure at the surface. As a result of breathing compressed air and being subjected to high pressures caused by water depth, the amount of nitrogen dissolved in blood and other tissues increases. If the diver returns to the surface too rapidly, the nitrogen forms bubbles in the blood as it becomes less soluble due to a decrease in pressure. The nitrogen bubbles can cause great pain and possibly death. To alleviate this problem somewhat, artificial breathing mixtures of oxygen and helium are used. Helium is only one-fifth as soluble in blood as nitrogen. As a result, there is less dissolved gas to form bubbles. Solution Concentrations Concentration: the amount (g) of solute dissolved in a specific amount of solvent 1. Concentrated: large amount of solute for the amount of solute. 2. Dilute: small amount of solute for the amount of solution. Methods of Determining Concentration of Solution: Concentration Ratios Concentration description Percent by mass Percent by volume Molarity Ratios mass of solute mass of solution x 100 volume of solute volume of solution x 100 moles of solute liter of solution Using Percent to Describe Concentration • Percent by mass usually describes solutions in which a solid is dissolved in a liquid. • Represents the ratio of the solute’s mass to the solution’s mass expressed as a percent. • The mass of solution equals the sum of the masses of the solute and solvent. Using Percent to Describe Concentration Calculating Percent by Mass In order to maintain a sodium chloride concentration similar to ocean water, an aquarium must contain 3.6 g NaCl per 100g of water. What is the percent by mass of NaCl in the solution? Find mass of the solution. Mass of solution = g solute + g solvent = 3.6g + 100g = 103.6 g Using Percent to Describe Concentration Calculating Percent by Mass In order to maintain a sodium chloride concentration similar to ocean water, an aquarium must contain 3.6 g NaCl per 100g of water. What is the percent by mass of NaCl in the solution? Substitute values into equation Percent by mass = mass of solute mass of solution x100 = 3.6g ÷ 103.6g x 100 =3.5% Using Percent to Describe Concentration Calculating Percent by Volume • describes solutions in which both the solute and solvent are liquids • Volume of the solution is the sum of the volumes of the solute and solvent Example: 70% isopropyl alcohol means that 70 volumes of alcohol are dissolved in 100 volumes of water, thus 30 volumes of water are in every 100 volumes of the isopropyl alcohol solution. Using Percent to Describe Concentration Calculating Percent by Volume What is the percent by volume of ethanol in a solution that contains 35mL of ethanol dissolved in 155mL of water? Volume of solution = vol. solute + vol. solvent = 35mL + 155mL = 190mL = 35mL x 100 190mL =18.4% Calculating Molarity • Molarity (M) is the number of moles of solute dissolved per liter of solution. • Also known as molar concentration. • A liter of solution containing one mole of solute is a 1M solution. • Formula: Molarity(M) = moles of solute liters of solution Calculating Molarity Example: A 100.5 mL intravenous (IV) solution contains 5.10g of glucose (C6H12O6). What is the molarity of this solution? [molar mass of glucose is 180.16 g/mol] Use molar mass to calculate the number of moles of C6H12O6. Round to 4 places after decimal. 5.10 g C6H12O6 1 mol C6H12O6 180.16 g C6H12O6 = .0283 mol C6H12O6 Calculating Molarity 2. Convert mL to L. 100.5mL x 1L = 0.1005 L solution 1000mL 3. Substitue the known values into the equation. Molarity = moles solute liters of solution M = 0.0283 mol C6H12O6 = 0.282 mol/L soln. 0.1005 L soln. = 0.28M Diluting Solutions Often stock solutions must be diluted from one concentration to another. How is the volume of the stock solution to be diluted determined? Rearrange the molarity equation and the number of moles solute does not change and we get the equation: M1V1 = M2V2 Diluting does Not add solute, Only solvent. Diluting Stock Solutions What volume, in milliliters of 2.00M calcium chloride solution would you use to make 0.50 L of 0.30M calcium chloride solution? Analyze the problem: what do you have and what are you looking for. Given Unknown M1 = 2.00M CaCl2 V1 = ? L M2 = 0.300M V2 = 0.50L Diluting Stock Solutions What volume, in milliliters of 2.00M calcium chloride solution would you use to make 0.50 L if 0.30M calcium chloride solution? Solve for the Unknown: rearrange the equation to solve for the unknown M1V1 = M2V2 V1 = M2 V2 M1 Diluting Stock Solutions What volume, in milliliters of 2.00M calcium chloride solution would you use to make 0.50 L if 0.30M calcium chloride solution? Substitute known values and solve. V1 = 0.50L x 0.300M 2.00M V1 = 0.075L x 1000mL 1L V1 = 75mL ( the question asked for mL!!!) Colligative Properties Physical properties of a solution that are affected by the number of particles in the solution. • Lowers vapor pressure: less gas escapes from the liquid because of the solute Colligative Properties • Raises boiling point: it takes more energy to overcome the attraction between the solute and solvent • Lowers freezing point: solute disrupts the solvent particles from forming a solid example: Antifreeze (ethylene glycol)