Hypotonic, Hypertonic and Isotonic
advertisement

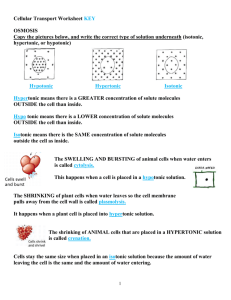
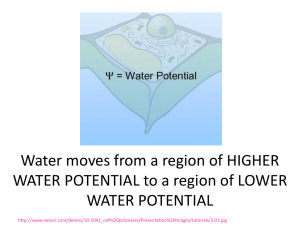
Hypotonic, Hypertonic and Isotonic How diffusion works in different solutions. Isotonic Solution Latin: “Iso” means equal. So… Isotonic means that it has the same solute concentration on the inside of the cell as the outside of the cell. Inside the Cell: 20% solute, 80% water Outside the Cell: 20% solute, 80% water Isotonic As a result, there is no net movement of water molecules. The concentration on both sides of the membrane remains the same. Inside the Cell: 20% solute, 80% water Outside the Cell: 20% solute, 80% water Hypertonic Solution Latin: “Hyper” means over or above. So… Hypertonic means there is a higher solute concentration on the outside of the cell, than on the inside of the cell. Inside the Cell: 20% solute, 80% water Outside the Cell: 40% solute, 60% water Hypertonic Solution Water particles are going to move OUT of the cell to even out the concentration. This causes the cell to shrivel. Hypotonic Solution Latin: “Hypo” means low or below. So… Hypotonic means that it has the lower solute concentration on the outside of the cell, than on the inside of the cell. Inside the Cell: 20% solute, 80% water Outside the Cell: 10% solute, 90% water Hypotonic Solution Water molecules are going to move into the cell, to even out the concentrations. The causes the cell to get larger. You Try 1. What is the % of water inside the cell? 2. What is the % of water outside the cell? 3. Will osmosis occur? If so, in what direction? 4. Will the cell shrink or swell? 5. This picture shows a cell in a ______________ solution. 10% glucose 20% glucose You Try 1. What is the % of water inside the cell? 2. What is the % of water outside the cell? 3. Will osmosis occur? If so, in what direction? 4. Will the cell shrink or swell? 5. This picture shows a cell in a ______________ solution. 20% glucose 20% glucose You Try 1. What is the % of water inside the cell? 2. What is the % of water outside the cell? 3. Will osmosis occur? If so, in what direction? 4. Will the cell shrink or swell? 5. This picture shows a cell in a ______________ solution. 40% glucose 20% glucose Real World Examples Why does my skin wrinkle in the bath? We are sitting in a hypotonic solution So water rushes in, causing our skin cells to swell Our swollen cells are too large to fit on our bodies, causing wrinkles to occur Why does a slug die when you pour salt on it? The slug is put in an intense hypertonic solution So water rushes out of the slug, causing it to lose water rapidly – thus, the slug dies Real World Examples Why can’t you put a salt-water fish in a fresh-water aquarium? The fish is put in a hypotonic solution. So water rushes in, causing the fish to gain too much water, which rupture’s the fish’s cells, causing it to die How can a fish even live in salt-water? Fish live in a hypertonic solution, so ordinarily water wants to rush out Fish have to drink lots of salt water to make the concentrations even – then they pump out the salt using active transport