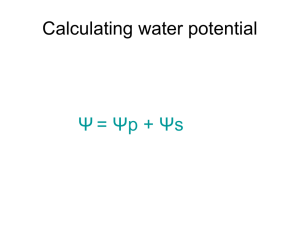Water potential
advertisement

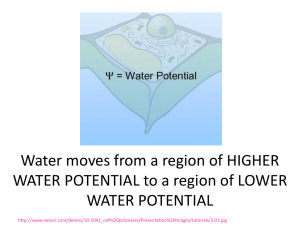
Water potential Water Potential, Ψ* , describes the likelihood water will diffuse in or out of a cell. Based on two factors: Ψ = Ψs+ Ψp • solute concentration gradient (aka solute potential) • pressure in the cell (aka pressure potential) • water moves from the sol’n with higher water potential to that with lower water potential *greek letter psi Solute Potential, Ψs (aka solute conc.) cell environs Ψ = Ψs+ Ψp • hypertonic Ψs in environs > in cell cell loses water via osmosis • hypotonic Ψs in environs < in cell Turgor pressure, bail, or burst • Here, cell solute potential has increased, thus cell water concentration decreased • Ψ of pure water = 0, so Ψs is always negative Pressure Potential, Ψp Ψ = Ψs+ Ψp • if pressure in cell increases, so does Ψ • living cells usually have positive Ψp (turgid, not flaccid) - pressure 0 pressure + pressure Sum of solute, pressure potentials yields water potential No solute, no pressure Net diffusion into cell No net diffusion Again: (aka solute potential) At 75, no pressure, but great solute gradient = net diffusion into cell Negative water potential At 100, pressure potential prevents continued solute diffusion despite solute potential = no net diffusion Zero water potential To quantify Ψ find solute potential: temperature Ψs = -iCRT K = C + 273 ionization factor sucrose does not ionize in water i = 1.0 pressure constant .0831 L bars/moles K molar concentration moles/L Ex: solute potential of a 1.0M sucrose sol’n at 22C at : std atmospheric pressure Ψs = -(1)(1.0)(.0831)(295) Ψs = -24.51bars Ψ = -24.51bars + Ψp Since water at atmospheric pressure has a pressure potential of zero, water pot. = solute pot. (+ 0) Ψ = -24.51bars