Uncertainty in Measurements
advertisement

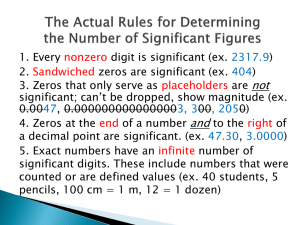
Uncertainty in Measurements Measurements always involve a comparison. When you say that a table is 6 feet long, you're really saying that the table is six times longer than an object that is 1 foot long. The foot is a unit; you measure the length of the table by comparing it with an object like a yardstick or a tape measure that is a known number of feet long. The comparison always involves some uncertainty. If the tape measure has marks every foot, and the table falls between the sixth and seventh marks, you can be certain that the table is longer than six feet and less than seven feet. To get a better idea of how long the table actually is , though, you will have to read between the scale division marks. This is done by estimating the measurement to the nearest one tenth of the space between scale divisions. Which of the following best describes the length of the beetle's body in the picture to the left? Between 0 and 2 in Between 1 and 2 in Between 1.5 and 1.6 in Between 1.54 and 1.56 in Between 1.546 and 1.547 in Uncertainty in Length Measurements • Measurements are often written as a single number rather than a range. The beetle's length in the previous frame was between 1.54 and 1.56 inches long. The single number that best represents the measurement is the center of the range, 1.55 inches. When you write the measurement as a single number, it's understood that the last figure (the 5 in this case) had to be estimated. Consider measuring the length of the same object with two different rulers. • Give the correct length measurement for the steel pellet for each of the rulers, as a single number rather than a range: Left Ruler: ................ in Right Ruler: .............. in ANSWERS • The left ruler has scale markings every inch, so you must estimate the length of the pellet to the nearest 1/10 of an inch. 1.4 in, 1.5 in, or 1.6 in would be acceptable answers. • The right ruler has scale markings every 0.1 inches, so you must estimate the length of the pellet to the nearest 0.01 inches. 1.46 inches is an acceptable answer. Uncertainty in Length Measurements • Give the correct length measurement for this electronic component for each of the rulers, as a single number rather than a range: Blue Ruler:................ cm White Ruler:.............. cm The blue ruler has scale markings every 0.1 cm, so you must estimate the length of the electronic component to the nearest 0.01 cm. 1.85 cm, 1.86 cm, or 1.87 cm would be acceptable answers. The white ruler has scale markings every 1 cm, so you must estimate the length of the electronic component to the nearest 0.1 cm. 1.9 cm is an acceptable answer. Uncertainty in Temperature Measurements • A zero will occur in the last place of a measurement if the the measured value fell exactly on a scale division. For example, the temperature on the thermometer just should be recorded as 30.0°C. Reporting the temperature as 30°C would imply that the measurement had been taken on a thermometer with scale marks 10°C apart! Uncertainty in Temperature Measurements • A temperature of 17.00°C was recorded with one of the three thermometers to the left. Which one was it? the top one the middle one the bottom one either the top one, or the middle one either the middle one, or the bottom one it could have been any of them ANSWERS • The top thermometer had scale markings 0.1°C apart; it could be read to the nearest 0.01°C. • The middle thermometer has scale markings ever 0.2°C, so it can be read to the nearest 0.02°C. • The bottom thermometer has markings every degree, and can be read to the nearest tenth of a degree. Try again. Uncertainty in Volume Measurements • Use the bottom of the meniscus (the curved interface between air and liquid) as a point of reference in making measurements of volume in a graduated cylinder, pipet, or buret. In reading any scale, your line of sight should be perpendicular to the scale to avoid 'parallax' reading errors. • The graduated cylinder on the left has scale marks 0.1 mL apart, so it can be read to the nearest 0.01 mL. Reading across the bottom of the meniscus, a reading of 5.72 mL is reasonable (5.73 mL or 5.71 mL are acceptable, too). Enter the volume readings for the middle and right cylinders below, assuming each scale is in mL. • Middle Cylinder Volume:................mL • Right Cylinder Volume:................mL ANSWERS • The middle cylinder has graduations every mL, and can be read to the nearest 0.1 mL. Since the meniscus touches the mark, the reading should be recorded as 3.0 mL, NOT as 3 mL. If you read 3.1 mL, you were probably reading across the top of the meniscus. Read at the bottom of the meniscus. • The right cylinder has graduations every 0.1 mL, and can be read to the nearest 0.01 mL. Since the meniscus is just below the halfway mark between 0.3 and 0.4, the reading should be recorded as 0.34 mL (although readings of 0.35 mL or 0.33 mL are acceptable). If you read 0.37 or 0.38, you were probably reading across the top of the meniscus. Read at the bottom of the meniscus. Exact Numbers • • • • Numbers obtained by counting have no uncertainty unless the count is very large. For example, the word 'sesquipedalian' has 14 letters. "14 letters" is not a measurement, since that would imply that we were uncertain about the count in the ones place. 14 is an exact number here. Very large counts often do have some uncertainty in them, because of inherent flaws in the counting process or because the count fluctuates. For example, the number of human beings in the state of Maryland would be considered a measurement because it can not be determined exactly at the present time. Numbers obtained from definitions have no uncertainty unless they have been rounded off. For example, a foot is exactly 12 inches. The 12 is not uncertain at all. A foot is also exactly 30.48 centimeters from the definition of the centimeter. The 8 in 30.48 is not uncertain at all. But if you say 1 foot is 30.5 centimeters, you've rounded off the definition and the rounded digit is uncertain. Which of the following quantities can be determined exactly? (Select all that are NOT measurements.) The number of light switches in the room you're sitting in now The number of ounces in one pound The number of stars in the sky The number of inches per meter The number of red blood cells in exactly one quart of blood ANSWERS • Anything that can be easily counted is exact. The number of light switches in the room you're sitting in now is exact, for example.Any defined quantity is exact. The number of ounces in one pound is exactly 16. The number of inches per meter must be exact since there are exactly 30.48 centimeters in a foot, exactly 12 inches in a foot, and exactly 100 centimeters in a meter. • Stars in the sky and red blood cells in a given volume of blood can be counted, but the counts are so large that there will inevitably be some uncertainty in the final result. What are Significant Digits? All of the digits up to and including the estimated digit are called significant digits. Consider the following measurements. The estimated digit is red: Measurement 142.7 g 103 nm 2.99798 x 108 m Number of Significant Digits 4 3 6 Distance between Markings onMeasuring Device 1g 10 nm 0.0001 x 108 m QUESTIONS A sample of liquid has a measured volume of 23.01 mL. Assume that the measurement was recorded properly. 1. How many significant digits does the measurement have? Enter your answer here:………….. 2. Suppose the volume measurement was made with a graduated cylinder. How far apart were the scale divisions on the cylinder, in mL? A) 10 mL B)1 mL C) 0.1 mL D) 0.01 mL E) not enough information 3. Which of the digits in the measurement is uncertain? A) "2" B) "3" C)"0" D)"1" E)not enough information ANSWERS 1. This measurement had FOUR significant figures. The 2, 3, and 0 are certain; the 1 is uncertain. Significant digits include all of the figures up to and including the first uncertain digit. 2. Since the hundredths place was uncertain, the graduated cylinder must have had markings 0.1 mL apart. 3. The last figure is the uncertain one; writing the number as 23.01 mL means: My best estimate of the volume is 23.01 mL, but it could have been 23.00 mL or maybe 23.02 mL. QUESTIONS A piece of steel has a measured mass of 1.0278 g. Assume that the measurement w as recorded properly. 1. How many significant digits does the measurement have? Enter your answer here: …………. 2. How far apart were the marks on the scale the mass was read from, in g? A) 1 g B) 0.1 g C) 0.01 g D) 0.001 g E) 0.0001 g F) 0.00001 g G) not enough information 3. Which of the digits in the measurement is uncertain? A) "1" B) "0" C) "2" D) "7" E) "8" F) not enough information ANSWERS 1. This measurement had FIVE significant figures. The 1, 0, 2, and 7 are certain; the 8 is uncertain. Significant digits include all of the figures up to and including the first uncertain digit. 2. Since the ten thousandths place was uncertain, the scale must have had markings 0.001 g (1 mg) apart. 3. The last figure is the uncertain one; writing the number as 1.0278 g means: My best estimate of the volume is 1.0278 g, but it could have been 1.0277 g or maybe 1.0279. Counting Significant Digits Moving the Decimal Point Doesn't Change Significant Figures Usually one can count significant digits simply by counting all of the digits up to and including the estimated digit. It's important to realize, however, that the position of the decimal point has nothing to do with the number of significant digits in a measurement. For example, you can write a mass measured as 124.1 g as 0.1241 kg. Moving the decimal place doesn't change the fact that this measurement has FOUR significant figures. Suppose a mass is given as 127 ng. That's 0.127 µg, or 0.000127 mg, or 0.000000127 g. These are all just different ways of writing the same measurement, and all have the same number of significant digits: three. Counting Significant Digits Moving the Decimal Point Doesn't Change Significant Figures If significant digits are all digits up to and including the first estimated digit, why don't those zeros count? If they did, you could change the amount of uncertainty in a measurement that significant figures imply simply by changing the units. If 0.00125 L has 3 significant digits, you know the uncertainty is about 1 part in 100 (1 part in 125, to be exact). If it had 6 significant digits, the uncertainty would only be about 1 part in a million. By not counting those leading zeros, you ensure that the measurement has the same number of figures (and the same relative amount of uncertainty) whether you write it as 0.00125 L, 1.25 mL, or 1250 µL. QUESTIONS Fill in the blanks in the following table. Measurement Number of Significant Figures 1. 0.000341 kg = 0.341 g = 341 mg 2. 12 mg = 0.000012 g = 0.000000012 kg 3. 0.01061 Mg = 10.61 kg = 10610 g ……………… ……………… ……………… ANSWERS 1. 0.000341 kg = 0.341 g = 341 mg. 341 mg clearly has 3 significant figures, and so must the same measurement written in kg or g. 2. 12 µg = 0.000012 g = 0.000000012 kg. 12 µg clearly has 2 significant figures, and so must the same measurement written in g or kg. 3. 0.01061 Mg = 10.61 kg = 10610 g. 10.61 kg clearly has 4 significant figures, and so must the same measurement written in g or Mg. Counting Significant Digits • • • • A Procedure for Counting Significant Digits How can you avoid counting zeros that serve merely to locate the decimal point as significant figures? Follow this simple procedure: Move the decimal point so that it is just to the right of the first nonzero digit, as you would in converting the number to scientific notation. Any zeros the decimal point moves past are not significant, unless they are sandwiched between two significant digits. All other figures are taken as significant. Counting Significant Digits Any zeros that vanish when you convert a measurement to scientific notation were not really significant figures. Consider the following examples. Converted to Scientific Significant Measurement Notation Figures 0.01234 kg 1.234 x 10-2 kg 4 Leading zeros (0.01234 kg) just locate the decimal point. They're never significant. 0.012340 kg 1.2340 x 10-2 kg 5 Notice that you didn't have to move the decimal point past the trailing zero (0.012340 kg) so it doesn't vanish and so is considered significant. 0.000011010 m 1.1010 x 10-5 m 5 Again, the leading zeros vanish but the trailing zero doesn't. 4 Ditto. 0.3100 m 3.100 x 10-1 m Counting Significant Digits Measure ment Converted to Scientific Notation 321,010,000 3.2101 x 106 miles miles 84,000 mg 8.4 x 104 mg Signific ant Figures 5 (at Ignore commas. Here, the least) decimal point is moved past the trailing zeros (321,010,000 miles) in the conversion to scientific notation. They vanish and should not be counted as significant. The first zero (321,010,000 miles) is significant, though, because it's wedged between two significant digits. 2 (at The decimal point moves past least) the zeros (84,000 mg) in the conversion. They should not be counted as significant. Counting Significant Digits Measure ment Converted to Scientific Notation 32.00 mL 3.200 x 101 mL 302.120 lbs 3.02120 x 102 lbs Signific ant Figures 4 The decimal point didn't move past those last two zeros. They are significant. 6 The decimal point didn't move past the last zero (302.120 lbs, so it is significant. The decimal point did move past the 0 between the two and the three, but it's wedged between two significant digits, so it's significant as well. All of the figures in this measurment are significant. QUESTIONS Fill in the blanks in the following table. Number of Measurement Significant Figures a) 1. 0.010010 g …………………. b) 10.00 g …………………. c) 1010010 g ………………….. ANSWERS a) 0.010010 g has 5 significant figures. The trailing zero is significant; the leading zeros are not. b) 10.00 g has 4 significant figures. The trailing zeros are significant. The decimal point does not move past them when the number is converted to scientific notation. c) 1010010 g has at least 6 significant figures. The trailing zero(s) can not be counted as significant. When are Zeros Significant? From the previous frame, you know that whether a zero is significant or not depends on just where it appears. Any zero that serves merely to locate the decimal point is not significant. All of the possibilities are covered by the following rules: Rule Examples (Significant figures are red) 1. Zeros sandwiched between two significant digits are always significant. 1.0001 km 2501 kg 140.009 Mg 2. Trailing zeros to the right of the 3.0 m decimal point are always significant. 12.000 µm 1000.0 µm (trailing zero is significant by rule 2; others by rule 1.) When are Zeros Significant? 3. Leading zeros are never significant. 0.0003 m 0.123 µm 0.0010100 µm (trailing zeros are significant by rule 2; sandwiched zeros by rule 1.) 4. Trailing zeros that all appear to the 3000 m LEFT of the decimal point can not be 1230 µm assumed to be significant. 92,900,000 miles QUESTIONS Fill in the blanks in the following table. Minimum Minimum Number of Number of Measurement Significant Figures Measurement Significant Figures a) 1010.010 g ....................... d) 32010.0 g ........................ b) 0.00302040 g ....................... e) 0.01030 g ........................ c) f) 101000 g ....................... 100 g ........................ ANSWERS a) 1010.010 g has 7 significant figures. The trailing zero is right of the decimal point and is significant; the zeros sandwiched between the ones are also significant. b) 0.00302040 g has 6 significant figures. The trailing zero are significant, since it is to the right of the decimal point. The leading zeros are not significant. The zeros that are sandwiched between nonzero digits are significant. c) 101000 g has at least 3 significant figures. The trailing zero(s) can not be counted as significant. d) 32010.0 g has 6 significant figures. The trailing zero is significant because it is to the right of the decimal point; all of the other zeros are sandwiched between two significant figures, so they're significant, too. e) 0.01030 g has 4 significant figures. The trailing zero(s) are significant because they are to the right of the decimal point. Zero(s) that are to the left of the first nonzero digit are NOT significant. f) 100 g has at least 1 significant figures. The trailing zero(s) can not be counted as significant. Rounding Off Often a recorded measurement that contains more than one uncertain digit must be rounded off to the correct number of significant digits. For example, if the last 3 figures in 1.5642 g are uncertain, the measurement should be written as 1.56 g, so that only ONE uncertain digit is displayed. Rules for Rounding Off Measurements 1. All digits to the right of the first uncertain digit have to be eliminated. Look at the first digit that must be eliminated. 2. If the digit is greater than or equal to 5, round up. 1.35343 g rounded to 2 figures is 1.4 g. 1090 g rounded to 2 figures is 1.1 x 103 g. 2.34954 g rounded to 3 figures is 2.35 g. 3. If the digit is less than 5, round down. 1.35343 g rounded to 4 figures is 1.353 g. 1090 g rounded to 1 figures is 1 x 103 g. 2.34954 g rounded to 5 figures is 2.3495 g. Try these a) b) c) 2.43479 rounded to 3 figures is ........... b)1.756243 rounded to 4 figures is ........... 9.973451 rounded to 4 figures is ........... Answers a) 2.43479 should be rounded to 2.43. The fourth figure is a 4, so the number is rounded down. b) 1.756243 should be rounded to 1.756. The fifth figure is a 2, so the number is rounded down. c) 9.973451 should be rounded to 9.973. The fifth figure is a 4, so the number is rounded down. Counting Significant Digits for a Series of Measurements • Suppose you weigh a penny several times and obtain the following masses. • 2.5019 g 2.5023 g 2.5030 g 2.5037 g 2.5043 g 2.5009 g • How many significant figures should the average mass be reported to? Counting Significant Digits for a Series of Measurements • The average can't be more precise than any of the individual measurements. The exact average is 2.5026833333333333333333... g. Clearly this is not an appropriate way to report the average, since it implies more precision than any of the masses being averaged actually have. Masses taken from the balance could be estimated to the nearest tenth of a milligram (0.0001 g) so the average can't be more precise than 2.5027 g. • The last figure is the one and only uncertain figure. Remember the definition of significant figures: all digits up to and including the first uncertain digit. Look at the penny weights above. Which digits are uncertain? The last two places change from measurement to measurement. If we want to write an average so that the last figure recorded is the first (and only) uncertain figure, it's best to write the average as 2.503 g.