What_is_Science
advertisement

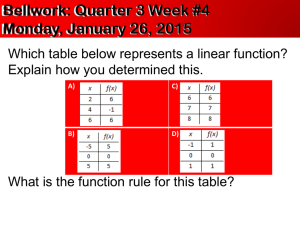
Introduction to Science Hypothesis_Variables_and Graphs.ppt Metric_Unit_Analysis.ppt Metrics_and_Measuring.ppt Scientific_Notation.ppt Sci_Not_Multiply_Divide.ppt Sci_Notation_Sig_Figures.ppt Scientific Modeling.ppt Scientific_Method.ppt Chemistry Introduction to Science Terms Pure Science A number without a unit is meaningless Technology Base Unit Scientific Models Prefix Scientific Laws meter Scientific Theories gram Inference Kelvin The Scientific Method second Observation mole Question liter Hypothesis 1 mL = 1 cm3 Experiment to test Hypothesis kilo- Analysis (draw Conclusions) centi- Testing only one variable at a time milli- Qualitative observations micro- Quantitative observations Derived Unit Unit Analysis 1 g H20 = 1 mL H2O = 1 cm3 H2O Significant Digits Scientific Notation Uncertainty in a Measurement Tables Graphs Line graphs X – Y Scatter Plot Bar graphs Circle graphs Introduction to Science Problem Solving Working with Unit Analysis Working with Significant Digits Working with Scientific Notation Working with Uncertainty in a Measurement Working with Tables Working with Graphs Introduction to Science Overall Concepts Understand how to properly use all the tools presented to collect and analyze data, and to infer from that scientific data, and be able to come to rational and valid conclusions based on that analysis and inference. Introduction to Science What is Science? Science is what scientists do Science is trying to explain the world around us Science is a way of thinking “Science is a system of knowledge based on facts or principles “social science” No, actually social studies Introduction to Science Branches of Science Science Biological Science Physical Science Earth Science Introduction to Science Branches of Science Science Biological Science Physical Science Earth Science Zoology Botany Ecology Science of living things Introduction to Science Branches of Science Science Biological Science Physics Chemistry Physical Science Earth Science Science of matter and energy Introduction to Science Branches of Science Science Biological Science Physical Science Earth Science Geology The systems of the earth Meteorology Astronomy Introduction to Science Branches of Science There are many more branches to the sciences The three categories overlap - Biochemistry - Astrobiology - Geophysics - etc. Introduction to Science Science and Technology Pure Science - search for scientific knowledge Technology – application of science The two are interrelated Technology develops new tools for investigating nature from past scientific knowledge (knowledge of photons leads to the invention of the laser) New scientific discoveries can occur because of new technology (lasers can be used for a number of pure scientific pursuits in physics and biology) Introduction to Science Scientific Modeling.ppt Reality and Scientific Models At its very core, science is the discipline of making models of reality that allow us to understand and make predictions about the world that we live in, the world around us. These “Scientific Models” are created from observations and/or experiments. This data from observations and experiments should always be from “actual reality” and not from opinions, bias, or virtual reality. Introduction to Science Scientific Modeling.ppt Reality and Scientific Models These models of “reality” are constantly tested and refined in the scientific community. In fact, the highest ideal of science is to prove a theory or law wrong. Conversely, if after repeated attempts to refute the theory or law, no one has proven it wrong, we can be fairly certain that it is probably right and can be used to make predictions. Introduction to Science Scientific_Method.ppt Scientific Laws Describes what happens Quantitative – use numbers and equations to describe Often equations are part of the law - (Mathematics is a universal language) Example: The Law of Gravity Introduction to Science Scientific_Method.ppt Scientific Theories A reasoned explanation tested by many observations and experiments (Scientific theories are a scientific model) Tells why things are Three things Must explain clearly and simply Must be repeatable (testable) Must be able to make predictions Introduction to Science Scientific_Method.ppt Scientific Theories Theories are generally conceived through the process called inference An inference is a logical interpretation based on data that scientists collect from both observations and experiments. Theories can be changed or modified by new evidence Example: The Cell Theory Introduction to Science Scientific_Method.ppt Law vs. Theory Law Theory Describes how Explains why Summarizes observations Agrees with observations Usually an equation Predicts new discoveries Introduction to Science Models A more specific terminology often used in science and technology is the general term “model”. A representation of some object or event Made to better understand it Often used if real thing is too big, small or complex. Introduction to Science Models Come in a variety of forms, almost always physical in nature. Physical models Diagrams Computer models An example might be a model car, a computer model of a building, etc. Introduction to Science Scientific_Method.ppt The Scientific Method A way of thinking about and solving problems It is a logical method You do it all the time Introduction to Science Scientific_Method.ppt The Scientific Method Starts with observation- can be anything Question – what do you want to know? Gather data- what is already known Form hypothesis- a possible explanation Design experiment to test hypothesis This is the hard part Introduction to Science Scientific_Method.ppt The Scientific Method Experiments generate more observations Allow us to analyze (draw conclusions) about hypothesis Support the hypothesis or not If not modify hypothesis Introduction to Science Scientific_Method.ppt Formulate Observe a question Experiment tests hypothesis Collect data Form hypothesis Observation Observation Observation Draw Conclusions Introduction to Science Scientific_Method.ppt Introduction to Science Scientific_Method.ppt The Scientific Method Does not always work this way, but gives a way of guiding our thinking Hard part is testing only one variable at a time. Changing only one thing at a time If you change more than one, you don’t know which one is the cause Metric System Metrics_and_Measuring.ppt Observations Qualitative – describe with words - Hot , red, large Quantitative – (requires measurements with numbers) describe with numbers - 100° , 10 meters, 3.46 grams Scientists prefer quantitative Easy to agree upon No personal bias Metric System Metrics_and_Measuring.ppt Measurement A number without a unit is meaningless It is 4 long 4 what? Scientists use the metric system or SI for le System Internationale d’Units Makes sharing data easier Metric System Metrics_and_Measuring.ppt Base Units and Prefixes A simple Measurement always has a Base unit and can have a prefix if needed Prefixes multiply or divide the base units by multiples 10 Prefixes are the same for all base units Metric System Metrics_and_Measuring.ppt Base Units Quantity Unit Abbreviation Length meter m Mass gram g Temperature Kelvin K Time second s Electric current ampere A Amount of Substance mole mol Luminous Intensity candela cd Volume liter L 1 mL = 1 cm3 Metric System Metrics_and_Measuring.ppt Prefixes Prefix Symbol Meaning As a Number Giga- G billion 1 000 000 000 Mega- M million 1 000 000 Kilo- k thousand 1 000 Hecto- h hundred 100 Deca- da ten 10 Deci- d tenth 0.1 Centi- c hundredth 0.01 Milli- m thousandth 0.001 Micro- μ millionth 0.000 001 Nano- n billionth 0.000 000 001 Metric System Metrics_and_Measuring.ppt Derived Units (Compound Units) A Derived Unit is a unit of measurement that is defined by a combination of base units (can even be the same base unit – cm3) Area – cm2 m2 Volume – cm3 m3 Density – g/cm3 Velocity – m/s etc. kg/L mm/ms etc. g/mL etc. etc. Metric System Metrics_and_Measuring.ppt Mass, also known as "matter".. (all the "stuff" in the universe) is the amount of matter an object contains measured in kilograms (kg). 1,000 grams = 1 kg Scientists measure an object's mass using a triple beam balance. Weight is related to Mass but is NOT the same thing. Weight is the mass multiplied by the force (acceleration) of gravity on the object. Mass is measured in a simple Unit – grams, kilograms, etc. Weight is measured in a derived Unit – kg m/s2 The mass of an object does not change, but it's weight can depending on the gravity- for example you weigh more on Earth than on the Moon because the Earth's gravity is much stronger. If I travel to the moon, my mass has not changed, but my weight has changed because the moon's gravity is less. Weight is measured in Newtons (N) because it's considered a force. Metric System Metrics_and_Measuring.ppt Metric_Unit_Analysis.ppt Unit Analysis (Dimensional Analysis) Unit Analysis is a method of using ratios equal to one (1) to change a starting unit measurement to a final unit measurement with a different unit label than what was started with. Metric System Metrics_and_Measuring.ppt A Special Situation in Metrics There is one special case in metrics that ties mass with volume. One gram of water, at temperature 4 °C is equal to one milliliter of water which is equal to one cm3 of water. 1 g H2O = 1 mL H2O = 1 cm3 H2O This case is only true for water! Measurements Metrics_and_Measuring.ppt Sci_Notation_Sig_Figures.ppt Significant Digits All measurements in science are written with the proper number of significant digits according to the type of measuring instrument used. These significant digits are added, subtracted, multiplied, and divided according to specific rules in order to keep the correct number of significant digits in the final answer. Measurements Scientific_Notation.ppt Sci_Not_Multiply_Divide.ppt Scientific Notation Sci_Notation_Sig_Figures.ppt A way of easily showing the significant digits in a number and also helping to handle very large or very small numbers is Scientific Notation. In this class, you will need to be able to manipulate the exponents without the help of a calculator. Measurements Metrics_and_Measuring.ppt Showing Uncertainty in a Measurement (recording the correct significant digits) When measuring, record the digits that are certain (you know these because there are marks or lines to use to determine the digits). Then, estimate a final digit between the marks that bracket the measurement. This is the correct measurement with the correct significant digits Tables and Graphs Hypothesis_Variables_and Graphs.ppt Tables - Organizing data into groups - Putting those groups into rows and columns - Gives us an easy way to compare data All tables that you make should be in the form shown in the PowerPoint above. Tables and Graphs Hypothesis_Variables_and Graphs.ppt Tables Tables and Graphs are made using the same components that are presented in the Scientific Method - Hypothesis - Control - Independent variable - Dependent variable Tables and Graphs Hypothesis_Variables_and Graphs.ppt Graphs Give a visual representation of data Summarizes data. Two types of variables Independent variable the thing you have control over Dependent variable the thing that you don’t have control over. The three basic types of graphs are line, bar, and circle Tables and Graphs Hypothesis_Variables_and Graphs.ppt Graphs All graphs include - A title - Labeled axes - A consistent scale. Tables and Graphs Hypothesis_Variables_and Graphs.ppt Line Graphs Line graphs - compare two variables. shows information that is connected in some way (such as change over time) 35 30 25 New York San Diego Salina Cruz 20 15 10 5 0 J F M A M J J A S O N D Tables and Graphs Hypothesis_Variables_and Graphs.ppt X –Y Scatter Plot A type of graph that displays values for two variables for a set of data. The data is displayed as a collection of points, each having the value of one variable determining the position on the horizontal axis and the value of the other variable determining the position on the vertical axis. Tables and Graphs Hypothesis_Variables_and Graphs.ppt Bar Graphs Bar Graphs - wide columns used for things like weight, height, and length. Compare quantities 12 10 8 6 4 2 0 Production of Energy per gram Carbohydrates Proteins Fats Tables and Graphs 33% Hypothesis_Variables_and Graphs.ppt 42% Circle Graphs Often called a pie chart divided into parts easy to compare to whole amount. Use several to show changes over time 25% Buildings Transportation Industrial