Scientific Notation
advertisement

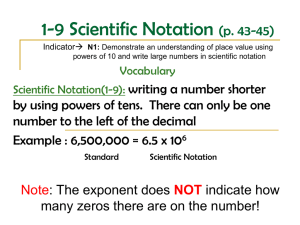
Watch “Powers of 10” http://micro.magnet.fsu.edu/primer/java/scienceopti csu/powersof10/ Scientific Notation - LARGE Numbers - POSITIVE Exponents Your pencil has 602,200,000,000,000,000,000,000 carbon atoms. this number in scientific notation. Write Remember, every whole number has a imaginary decimal point at the end. 602,200,000,000,000,000,000,000 • +23 6•0 2 2 x 1 0 1. Find the first non-zero number ... BASE NUMBER x POWER OF TEN ... and the last non-zero number. 2. Write that number underneath. 3. Put a decimal after the first digit (6). Your pencil has 6.022 x 10 atoms. 23 * This is the BASE NUMBER 4. Multiply the base number by the POWER OF TEN . 5. Count the number of decimal places from your NEW decimal point 6. to your OLD decimal point. Write that number as an exponent of 10. Scientific Notation - SMALL Numbers - NEGATIVE Exponents The radius of an atom is about 0.0000000005 m. Express in scientific notation. 0.0000000005 • –10 x 10 5 1. Find the first non-zero number ... ... and the last non-zero number. 2. Write that number underneath. 3. Put a decimal after the first digit (5). * This is the BASE Since it’s just a whole number, get rid of the decimal. NUMBER It’s negative because the number is really small. 4. Multiply the base number by the POWER OF TEN 5. Count the number of decimal places from your NEW decimal point 6. to your OLD decimal point. Write that number as an exponent of 10. The radius of an atom is 5 x 10 -10 m. Changing Scientific --> Standard Form WritingNotation Numbers in Standard Form EXAMPLE 2 Convert each value from scientific to standard notation 7 000 x 10 3 1. The exponent will tell you how many spaces to move the decimal. Remember, every number has an imaginary decimal point after it. 7 0 0 0 4.398 x 10 8 –4 2. Since the exponent is +3 –7 ,move +8 the decimal 4 7 83 spaces to the right. left 439,800,000 6.001 x 10 –4 .0 0 0 6001 3 x 10 .0 0 0 0 0 0 3 –7 3. Fill the empty place values with zeros.