Significant Figures
advertisement

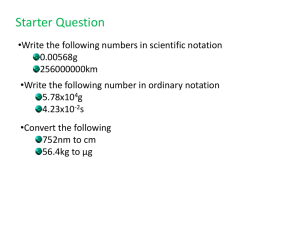
+ Significant Figures + Recording a Measurement Using Significant Digits: When recording a measurement, include every digit that is absolutely certain plus the first digit that must be estimated (guessed). This is the definition of a significant digit or significant figure. + Rules The following rules are used to determine the number of significant digits in a given measurement. 1. All non-zero digits are significant. Ex: 374 (___ sig. figs) 8.1 (___ sig. figs) 2. All zeroes between non-zero digits are significant. Ex: 50407 (___ sig. figs) 8.001 (___ sig. figs) + Practice Determine the number of sig. figs for the following: a) 2 b) 987 c) 56487209 d) 506973 e) 90003 + Rules con’t 3. Leading zeroes in a decimal are not significant. Ex: 0.54 (__ sig figs) 0.0098 (__ sig figs) 4. Trailing zeroes are significant if they are to the right of a decimal point. Ex: 2370 (__ sig figs) 16000 (__ sig figs) 16000.0 (__ sig figs) + Practice Determine the number of sig. figs for each value given. a) 0.54 = __________ sig figs b) 0.0098 = __________ sig figs c) 2370 = __________ sig figs d) 16070 = __________ sig figs e) 160.0 = __________ sig figs f) 37000 = __________ sig figs + Rules con’t 5. *In numbers greater than 1, trailing zeroes are not significant unless stated so.* Ex: Determine the number of sig. figs. a)Approximately pep rally. b)The 37000 students attended the beaker contained 37000 grams of Copper. c)37000 + Scientific Notation The last three zeroes may or may not be part of the measurement. To show that they are, we use scientific notation. All the zeroes written in the number in scientific notation are significant. 37000 with 3 sig. figs would be 37000 with 4 sig. figs would be 37000 with 5 sig. figs would be 37000 with 6 sig. figs would be + Practice Determine the number of sig. figs. for each value given. a) 5.80 x 104 =__________ sig figs b) 4.6800 x 104 =__________ sig figs c) 258000.0 =__________ sig figs + Practice Round each of the following to 3 sig figs a) 5.8467 x 104 b) 458900 c) 258000.0 d) 784643 e) 45.097 f) 0.00086432 g) 0.06598 + Homework Significant Figures Worksheet + Trick: Pacific-Atlantic Rule Here is an alternate rule for determining significant digits. The rule is really a mnemonic device.You, the student, are easily confused about the number of significant digits, especially if zeroes are present. This rule will allow you to achieve success in working with significant digits. This method is called the ”Pacific-Atlantic" method. If the number in question does not contain a decimal, think "A" for Absent. If the number in question does contain a decimal, think "P" for Present. Next, imagine a map of North America with north pointing to the top of the page. The "A" now stands for Atlantic and the "P" now stands for Pacific oceans. Now, imagine an arrow starting from the correct coast being drawn towards the number. Once the arrow hits a non-zero digit, that digit and all digits after it are significant. + Examples: How many significant digits are shown in the number? a)37 500 b)0.040500 + Addition and Subtraction: General Rules: 1. Add or subtract as normal. 2. Count the number of digits to the right of the decimal. 3. The answer must be rounded to contain the same number of decimal places as the value with the LEAST number of decimal places. *If there are no decimals then round to the number that is the least accurate* + Example: Perform the following calculations. a) 12.0 + 131.56 + 0.2798 = b) 135 + 45 + 0.3804 = c) 580 + 26.7 + 0.889 = d) 1000 – 8900 + 98.8 = + Multiplication and Division General Rules: 1. Multiply or divide as normal. 2. Count the number of sig figs. to each number. 3. The answer must be rounded to contain the same number of sig figs. as the number with the LEAST number of sig figs. Example 1. Perform the following calculation. 51.3 x 13.75 = ? Example 2. 3.0×1012 ÷ 6.02×1023 = ? + Scientific Notation Expresses numbers as a multiple of two factors: a number between 1 and 10 and ten raised to a power, or exponent. The exponent tells you how many times the first factor must be multiplied by 10 (i.e. how many places to move the decimal point, if there is no decimal point place it at the end of the value) + Convert into scientific notation a) 1 392 000 b) 0.000 000 028 c) The mass of a proton is 0.000 000 000 000 000 000 000 000 001 672 62kg. d) The mass of an electron is 0.000 000 000 000 000 000 000 000 000 000 910 939 kg. Why do you think we use scientific notation? + Multiplication and Division using Scientific Notation Multiplication • Multiply the first factors. • Add the exponents. a) (2 x 103) X (3 x 102) = Division • Divide the first factors. • Subtract the exponents. Dividend (top) – Divisor (bottom) b) (9 x 108) / (3 x 104) = + Homework Textbook Pg 32 #12-14 Pg 33 #15, 16 + Homework Significant Figures Review Worksheet + Sig Figs Test