Powerpoint 1
advertisement

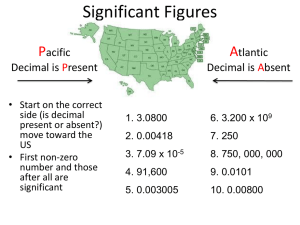
Measurement Measurement – a quantity that has both a number and a unit. • Measurements are fundamental to the experimental sciences • Units typically used in the sciences are the International System of Measurements (SI) Scientific Notation In science, we deal with some very LARGE numbers: 1 mole = 602000000000000000000000 In science, we deal with some very SMALL numbers: Mass of an electron = 0.000000000000000000000000000000091 kg Scientific Notation Imagine the difficulty of calculating the mass of 1 mole of electrons! 0.000000000000000000000000000000091 kg x 602000000000000000000000 ??????????????????????????????????? Scientific Notation: A method of representing very large or very small numbers in the form: M x 10n M is a number between 1 and 10 n is an integer . 2 500 000 000 9 8 7 6 5 4 3 2 1 Step #1: Insert an understood decimal point Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10n 2.5 x 9 10 The exponent is the number of places we moved the decimal. 0.0000579 1 2 3 4 5 Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10n 5.79 x -5 10 The exponent is negative because the number we started with was less than 1. Review: Scientific notation expresses a number in the form: M x 1 M 10 n 10 n is an integer Nature of Measurement Measurement - quantitative observation consisting of 2 parts • Part 1 - number Part 2 - scale (unit) Examples: 20 grams 6.63 x 10-34 Joule seconds Uncertainty in Measurement A digit that must be estimated is called uncertain. A measurement always has some degree of uncertainty. Precision and Accuracy Accuracy – measure of how close a measurement comes to the actual or true value of whatever is being measured. Precision – measure of how close a series of measurements are to one another. Neither accurate nor precise Precise but not accurate Precise AND accurate Why Is there Uncertainty? Measurements are performed with instruments No instrument can read to an infinite number of decimal places Which of these balances has the greatest uncertainty in measurement? Determining Error Accepted Value – the correct value based on reliable references Experimental Value – the value measured in the lab Error(can be +or-)=experimental value – accepted value Percent error = absolute value of error x 100% accepted value Significant Figures in Measurement In a supermarket, you can use the scales to measure the weight of produce. If you use a scale that is calibrated in 0.1 lb intervals, you can easily read the scale to the nearest tenth of a pound. You can also estimate the weight to the nearest hundredth of a pound by noting the position of the pointer between calibration marks. Significant Figures in Measurement If you estimate a weight that lies between 2.4 lbs and 2.5 lbs to be 2.46 lbs, the number in this estimated measurement has three digits. The first two digits (2 and 4 ) are known with certainty. The rightmost digit (6) has been estimated and involves some uncertainty. Significant Figures in Measurement Significant figures in a measurement include all of the digits that are know, plus a last digit that is estimated. Measurements must always be reported to the correct number of significant figures because calculated answers often depend on the number of significant figures in the values used in the calculation. Rules for Counting Significant Figures Nonzero integers always count as significant figures. 3456 m has 4 sig figs. Rules for Counting Significant Figures Leading zeros NEVER count as significant figures. 0.0486 cm has 3 sig figs. Rules for Counting Significant Figures Captive zeros (ones that are being hugged by sig figs) always count as significant figures. 16.07 mm has 4 sig figs. Rules for Counting Significant Figures Zeros at the end of a number are significant only if you can see the decimal point. 9.000 m has 4 sig figs 1050.0 mm has 5 sig figs Rules for Counting Significant Figures Zeros at the end are not significant if you can’t see the decimal point. 7000 m has 1 sig fig 27210 m has 4 sig figs Rules for Counting Significant Figures Exact numbers have an infinite number of significant figures. 1 meter = 100 cm Sig Fig Practice #1 How many significant figures in each of the following? 1.0070 m 5 sig figs 17.10 kg 4 sig figs 100,890 L 5 sig figs 3.29 x 103 s 3 sig figs 0.0054 cm 2 sig figs 3,200,000 m 2 sig figs Questions 1) 78ºC, 76ºC, 75ºC 2) 77ºC, 78ºC, 78ºC 3) 80ºC, 81ºC, 82ºC The sets of measurements were made of the boiling point of a liquid under similar conditions. Which set is the most precise? Set 2 – the three measurements are closest together. What would have to be known to determine which set is the most accurate? The accepted value of the liquid’s boiling point Questions How do measurements relate to experimental science? Making correct measurements is fundamental to the experimental sciences. How are accuracy and precision evaluated? Accuracy is the measured value compared to the correct values. Precision is comparing more than one measurement. Questions Why must a given measurement always be reported to the correct number of significant figures? The significant figures in a calculated answer often depend on the number of significant figures of the measurements used in the calculation. How does the precision of a calculated answer compare to the precision of the measurements used to obtain it? A calculated answer cannot be more precise than the least precise measurement used in the calculation. Question Determine the number of significant figures in each of the following 11 soccer players unlimited 0.070 020 meter 5 10,800 meters 3 5.00 cubic meters 3