Uncertainty in Measurement: Significant Figures
advertisement

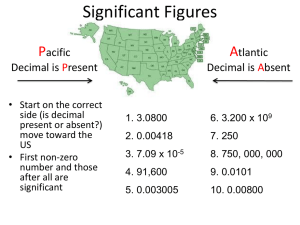
Every measurement we make includes some uncertainty. We can never measure something exactly or know a quantity with absolute certainty. The numbers (quantity) we use must tell us two things: 1. How large or small 2. How well were you able to measure it The digits we record in a measurement (certain and uncertain) are called, significant figures (sig. figs). The greater the # of sig. figs in a measurement, the greater the certainty. In general, all digits are significant, except zeros that are not measured but are used to position the decimal point (place holders). • Leading zeroes never count as sig. figs There are only 3 sig. figs in the quantity 0.00275 kg. • Internal zeroes always count as sig. figs The quantity 1.004 g has 4 sig. figs • Trailing zeroes count as sig. figs only if the decimal point is written. The quantity 12.40 mL has 4 sig. figs, but the quantity 250 mL has only 2 sig. figs. How many sig. figs are in… 12.35 g Answer 0.00568 L 3 (leading zeroes never count) 3.007 g 4 (internal zeroes always count) 21.0 °C 3 (trailing zeroes count if decimal is showing) 500 mL 1 (trailing zeroes do NOT count if no decimal is showing –– but don’t leave them out, or it looks like 5 mL!) 3 (leading zeroes never count, but trailing zero counts if decimal is showing) 0.0250 L 4 (count all non-zero digits, not just decimal places) Sig. Figs Sig. Figs a. 0.103 cm i. 2, 300 g b. 2.306 in j. 8.10 L c. 21 k. 2.40603 x 105 µm d. 0.032 mL l. 0.000200 kg e. 1000 mL/L m. 144 f. 100. Lbs n. 1001 tons g. 85 boxes o. 340. lbs Answers to calculation cannot be more accurate than the information you entered in calculation- but calculators don’t know that. 2 rules when reporting the uncertainty in calculations. Addition and Subtraction Division and Multiplication When adding or subtracting, round off to the fewest number of decimal places. 22.9898 g 1.00794 g 12.011 g 47.9982 g 84.00694 g, round to 5 sig. figs 84.007 g Keep the same number of sig. figs. as the measurement with the least number of sig. figs Example : 1.2m X 2.00m = 2.4 m The first measurement 1.2 has 2 sig. figs The second measurement has 3 sig. figs. So your answer may only have 2 sig. figs 1.234g + 2.2g + 3.45g = 2.2m X 333m = 47.0 m 2.2 s = 4.257 kg x (1019 m2 – 40 m2) (54.5 s x 31.3 s) 6.9 g 7.3 X 102 -You have to change the number to scientific notation because that is the only way you can have two sig. figs 21 m/s 2.44 kg·m2/s2 You’ve observed the changes that occur when you place a piece of Al foil into a blue solution. Lots of observations (avoid jumping to conclusions) Bubbles form (gas behaves like H2 gas) You’ve observed the relationship between P and V Best to quantify observations (measured volumes while applying pressure) PV = constant (1662 Robert Boyle- Boyle’s Law) Boyle’s Law describes what gases do, but not why. To answer the “why” we need a model. Imagine air as a collection of particles (tinyping pong balls) bouncing around inside syringe. Tiny particles = molecules Every time a molecule hits the syringe wall or plunger, it pushes against surface. The surface pushes back and molecule bounces off in another direction. This process is called gas pressure. Now, let’s say we decrease the volume of the syringe. What happens to the molecules inside the syringe ? They move! Smaller volume = more collisions = more gas pressure This moving-particles model of gases is called the kinetic molecular theory of gases. You bet! Here are some examples: Inflating a bike tire Inflating a balloon All gases obey Boyle’s Law and KMT of gases seems to explain gas pressure behavior for all gases. Absolutely not! Think gas splint test. Example: CO2 extinguishes flame Different gases= different molecules (particles are always moving and bouncing around, PV relationship is the same) Now, the question is what happens when different kinds of gases are combined?